Abstract
Purpose
Deproteinized bovine bone substitutes are commonly used in dental regenerative surgery for treatment of alveolar defects. In this study, three different bovine bone minerals - OCS-B (NIBEC, Seoul, Korea), Bio-Oss (Geistlich - Pharma, Switzerland), Osteograft/N - 300 (OGN, Dentsply Friadent Ceramed. TN, USA) - were investigated to analyze the basic characteristics of commercially available bone substitutes.
Methods
Their physicochemical properties were evaluated by scanning electron microscopy, energy dispersive X-ray spectrometer (EDS), surface area analysis, and Kjeldahl protein analysis. Cell proliferation and alkaline phosphatase (ALP) activity of human osteosarcoma cells on different bovine bone minerals were evaluated.
Results
Three kinds of bone substitutes displayed different surface properties. Ca/P ratio of OCS - B shown to be lower than other two bovine bone minerals in EDS analysis. Bio-Oss had wider surface area and lower amount of residual protein than OCS - B and OGN. In addition Bio-Oss was proved to have lower cell proliferation and ALP activity due to lots of residual micro particles, compared with OCS - B and OGN.
Conclusions
Based on the results of this study, three bovine bone minerals that produced by similar methods appear to have different property and characteristics. It is suggested that detailed studies and quality management is needed in operations for dental use and its biological effects on new bone formation.
Figures and Tables
Figure 1
Scanning electron micrographs of OCS-B (a, b, c), Bio-Oss (d, e, f) and OGN (g, h, i) at magnifications of ×100 (a, d, g), ×1000 (b, e, h) and ×3000 (c, f, i). Each bone substitute displayed characteristic surface structure.
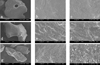
Figure 2
Cell proliferation measured at 1, 4 and 7 days of culture (n = 4 per each group). Polystyrene was significantly increased in optical density compared to OCS-B, Bio-Oss and OGN at 1, 4 and 7 days (p < 0.05). OCS-B was significantly increased in optical density compared to Bio-Oss at 4 and 7 days(p < 0.05). Bio-Oss was significantly decreased in optical density compared to OGN at 4 and 7 days (p < 0.05).

References
1. Borstlap WA, Heidbuchel KL, Freihofer HP, Kuijpers-Jagtman AM. Early secondary bone grafting of alveolar cleft defects: A comparison between chin and rib grafts. J Craniomaxillofac Surg. 1990. 18:210–205.
2. Tayapongsak P, Wimsatt JA, LaBanc JP, Dolwick MF. Morbidity from anterior ilium bone harvest : A comparative study of lateral versus medial surgical approach. Oral Surg Oral Med Oral Pathol. 1994. 78:296–300.
3. Clavero J, Lundgren S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin Implant Dent Relat Res. 2003. 5:154–160.

4. Emmings FG. Chemically modified osseous material for the restoration of bone defects. J Periodontol. 1974. 45:385–390.

5. Froum SJ, Thaler R, Scopp IW, Stahl SS. Osseous autografts. I. Clinical responses to bone blend or hip marrow grafts. J Periodontol. 1975. 46:515–521.

6. Mellonig JT. Autogenous and allogeneic bone grafts in periodontal therapy. Crit Rev Oral Biol Med. 1992. 3:333–352.

7. Rosenberg E, Rose LF. Biologic and clinical considerations for autografts and allografts in periodontal regeneration therapy. Dent Clin North Am. 1998. 42:467–490.
8. Wolff LF. Guided tissue regeneration in periodontal therapy. Northwest Dent. 2000. 79:23–28.
9. Schmitt JM, Buck DC, Joh SP, Lynch SE, Hollinger JO. Comparison of porous bone mineral and biologically active glass in critical-sized defects. J Periodontol. 1997. 68:1043–1053.

10. Mangano C, Bartolucci E, Mazzocco C. A new porous bydroxyapatite for promotion of bone regeneration in maxillary sinus augmentation: clinical and histologic study in humans. Clin Oral Implants Res. 2003. 18:23–30.
11. Wiltfang J, Schlegel KA, Schultze-Mosgau S, et al. Sinus floor augmentation with β-tricalciumphosphate(β-TCP): dose platelet-rich plasma promote its osseo-intergration and degradation. Clin Oral Implants Res. 2003. 14:213–218.

12. Barnett JD, Mellonig JT, Gray JL, Towle HJ. Comparison of freeze-dried bone allograft and porous hydroxyapatite in human periodontal defects. J Periodontol. 1989. 60:231–237.

13. Mellonig JT. Freeze-dried bone allografts in periodontal reconstructive surgery. Dent Clin North Am. 1991. 35:505–520.
14. Schllohorn RG, McClain PK. Combined osseous composite grafting, root conditioning and guided tissue regeneration. Int J Periodontics Restorative Dent. 1998. 8:8–30.
15. Gross J. Bone grafting material for dental application: A practical guide. Compendium. 1997. 18:1013–1036.
16. Slotte C, Lundgren D. Augmentation of calvarial tissue using non-permeable silicone domes and bovine bone mineral. An experimental study in the rat. Clin Oral Implants Res. 1999. 10:468–476.

17. de Bruijn JD, Klein CP, de Groot K, van Blitterswijk CA. The ultrastructure of the bone-hydroxyapatite interface in vitro. J Biomed Mater Res. 1992. 26:1365–1382.
18. Rosen BV, Hobbs LW, Spector M. Babbush CA, editor. The ultrastructure of anorganic bovine bone and selected synthetic hydroxyapatite used Bone:present and future. Dental implants: the art and science/edited by Babbush CA. 2001. Philadelphia: W.B. Sanduers Company;70.
20. Yildirim M, Spiekermann H, Handt S, Edelhoff D. Maxillary sinus augmentation with the xenograft Bio-Oss and autogenous intraoral bone for qualitative improvement of the implant site: a histologic and histomorphometric clinical study in humans. Int J Oral Maxillofac Implants. 2001. 16:23–33.
21. Mellonig JT. Human histologic evaluation of a bovine-derived bone xenograft in the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent. 2000. 20:19–29.
22. Harris RJ. Human histologic evaluation of a bone graft combined with GTR in the treatment of osseous dehiscence defects: a case report. Int J Periodontics Restorative Dent. 2000. 20:510–519.
23. Wetzel AC, Stich H, Caffesse RG. Bone apposition onto oral implants in the sinus area filled with different frafting materials. Clin Oral Implants Res. 1995. 6:155–163.

24. Klinge B, Alberius P, Isaksson S, Jonsson J. Osseous response to implanted natural bone mineral and synthetic hydroxylapatite ceramic in the repair of experimental skull bone defects. J Oral Maxillofac Surg. 1992. 50:241–249.

25. Wheeler SL, Holmes RE, Clahoun CJ. Six-year clinical and histologicstudy of sinus-lift grafts. Int J Oral Maxillofac Implants. 1996. 11:26–34.
26. Berglundh T, Lindhe J. Healing around implants placed in bone defects treated with Bio-Oss. An experimental study in the dog. Clin Oral Implants Res. 1997. 8:117–124.

27. Skoglund A, Hising P, Young C. A clinical and histologic examination in humans of the osseous response to implanted natural bone mineral. Int J Oral Maxillofac Implants. 1997. 12:194–199.
28. Valentini P, Bbensur D, Densari D, Graziani JN, Hammerle C. Histological evaluation and implantation procedure. A human case report. Clin Oral Implants Res. 1998. 9:59–64.
29. Eisenbarth E, Velten D, Muller M, Thull R, Breme J. Biocompatibility of beta-stabilizing elements of titanium alloys. Biomaterials. 2004. 25:5705–5713.

30. Piattelli M, Favero GF, Scarano A, Orsini G, Piattelli A. Bone reactions to anorganic bovine bone used insiuns lifting procedure: a histologic long-term report of 20 cases in man. Int J Oral Maxillofac Implants. 1999. 14:835–840.
31. Norton MR, Odell EW, Thompson ID, Cook RJ. Efficacy of bovine bone mineral for alveolar augmentation: a human histologic study. Clin Oral Implants Res. 2003. 14:775–783.

32. Buser D, Bragger U, Land NP, Nyman S. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin Oral Implants Res. 1990. 1:22–32.

33. Hammerle CHF, Olah AJ, Schmid J, et al. The biological effect of deproteinized bovine bone on bone neoformation on the rabbit skull. Clin Oral Implants Res. 1997. 8:198–207.
34. Park JW. Evaluation of deproteinized bovine bone mineral as a bone graft substitute : A comparative analysis of basic characteristics of three commercially available bone substitutes. J Korean Acad periodontol. 2005. 35:863–875.

35. Park JB, Han SH, Kim KH, et al. Evaluation on the bone regenerative capacity of deproteinized bovine bone-derived bone graft material(OCS-B). The Journal of the Korean Dental Association. 2006. 44:359–366.
36. Storgard-Jensen S, Aaboe M, Pinholt EM, et al. Tissue reaction and material characteristics of four bone substitutes. Int J Oral Maxillofac Implants. 1996. 11:55–66.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download