Cardiac transplantation is the only therapy available for terminal heart failure, and was pioneered by Christian Barnard in the 1970's, who also conducted the first heart transplant in 1976, although unfortunately this patient expired 18 days after surgery1). However, subsequently, many immunosuppressive agents and operation techniques were developed. In particular, the increased survival rates being achieved today can be attributed to a number of factors, such as, the refinement of medical and surgical techniques, better patient selection criteria, improved myocardial preservation, and the judicious use of cyclosporine, a selective T-cell immunosuppressant. Today more than 2000 heart transplants are performed annually, and in 2008 Roussel et al. reported survival rates after 5, 10, and 15 year postop of 75, 58, and 42%, respectively2). In the Republic of Korea, the first cardiac transplantation was performed at 1992, and in the 2008 the summed total number of cases performed stood at 434; 20~30 cases were performed annually until 2006 but this increased to 50 cases in 2007 and to 84 cases in 2008.
On the other hand, orthopaedic complications, such as osteopenia, osteoporosis, fractures, and osteonecrosis are also increasing in parallel with survival rates and the use of immunosuppressants. In particular, the incidence of osteonecrosis of the femoral head has been reported to be 2~3%3). This condition has been well documented after renal transplantation, but few reports have addressed osteonecrosis following cardiac transplantation, although it was been first reported by Danzig, et al. as long ago as 19764).
Because patients are invariably administered adrenocortical hormones and immunosuppressive agents after cardiac transplantation, they are at high risk of infection and often develop systemic complications, such as, an electrolyte imbalance. Furthermore, adrenal insufficiency, caused by suppression of the hypophysis-adrenocortical axis after adrenocortical hormone administration, increases the risk of surgery and anesthesia. In particular, anesthesia poses obvious risks for patients with a denervated heart. Authors report a satisfactory outcome for total hip arthroplasty for osteonecrosis of the femoral head after cardiac transplantation.
Case Report
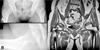
A 49-year-old woman visited our institution with pain in both hips with right side dominance of 3 weeks duration. She had undergone cardiac transplantation due to dilated cardiomyopathy six years previously and had administrated 4 mg of methylprednisone, 250 mg mycophenolic acid, and 8 mg of tacrolimus per day for immunosuppressive purposes. She had a history of medical treatment for repeated pyogenic pneumonia and hyperkalemia. At this presentation, she had a severe limping gait, which apparently tended to aggravate. Furthermore, simple radiography revealed a large synovial pit at the right femoral neck, and MRI showed osteonecrosis of both femoral heads (Fig. 1). 2D-echocargiography was performed preoperatively. The results were normal left ventricular chamber size, normal wall thickness, and moderate LV systolic function (ejection fraction = 55%) with anterior septal hypokinesia. Cardiovascular department doctor recommened us to monitor cardio-pulmonary status carefully.
Total hip arthroplasty with MIS-2-incision technique was performed on the right hip. Briefly, with the patient in a left lateral position, the joint capsule was exposed and the femoral head removed through an anterior incision placed between the tensor fascia lata and gluteus medius. An acetabular cup was inserted after reaming the acetabulum. The joint capsule was exposed by a posterior incision through gluteus maximus fibers and femoral stem was inserted after femoral reaming. The femoral neck was then turned to the anterior side, and the artificial femoral head was inserted and reduced. A Delta PF® (Lima) acetabular cup and an M/L Taper® (Zimmer, U.S.A.) femoral stem were used without cement fixation. The patient was admitted to aseptic room for 48 hours postoperatively, according to medical consult with careful cardio-pulmonary status monitoring. Prophylactic 1st generation cephalosporin antibiotic was administered (one dose of one gram preoperatively and three doses of one gram during the first day after operation) and immunosupressors which was administrated was continued. The patient started partial weight bearing ambulation with a walker on the first day after surgery and crutch ambulation at 1 week. On POD 10, she was discharged without any particular complication.
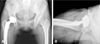
At her 26-month follow-up, she was ambulated without support. Furthermore, she was able to perform all daily activities without difficulty, and her Harris Hip Score had improved from 46 points preop to 98 points at last follow-up(Fig. 2).
Discussion
Avascular necrosis was reported to take place in 2~3% of cardiac transplant recipient3). Pre-exciting multifactorial metabolic bone disease, low body mass index and muscle mass, postoperative malnutrition and inactivity as well as the immunosuppressive therapy such as high doses of corticosteroids may all lead to poor bone quality and increased risk of osteoarticular disease in cardiac transplant recipients. Etiology of osteonecrosis after cardiac transplantation has still not been clearly elucidated, but the consensus of the studies performed to date shows that it is associated with adrenocortical hormones. A close relation between adrenocortical hormones and osteonecrosis is suggested by the observation that the incidence of osteonecrosis decreases from 17% to 1% when treatment regimen improvements justified minimizing the administration of adrenocortical hormones after renal transplantation, and by the high incidence of osteonecrosis in patients with systemic lupus erythematosus or Cushing's syndrome, who take adrenocortical hormones continuously. Fisher et al. reported that in experimental animals hypercholesterolemia can occur after the administration of massive doses of adrenocortical hormones, and that this induces fat embolism in bones and subsequent avascular necrosis due to small artery occlusion5). Li et al. showed that adrenocortical hormones cause stem cells in bone marrow to differentiate to fat cells and that this reduces vascularization6). On the other hand Korompilias et al. concluded that adrenocortical hormones are trigger factors and that immune reactions play an important role in pathogenesis of avascular osteonecrosis7). Bradbury et al. reported that osteonecrosis develops on average at 5 months (range 2~12 months) after cardiac transplantation3) and Leon et al. reported that the time interval from the heart transplantation to the presentation of avascular necrosis ranged between 7 and 15 months (average 11.3 months8)). Similar results have been reported after renal transplantation, which again supports the hypothesis that adrenocortical hormones induce immune reactions and that these cause avascular osteonecrosis.
Daily and maximum dose are known to be more important factors of osteonecrosis development than dose duration or accumulation. Bradbury et al. concluded that osteonecrosis is associated with maximum dose, rather than with accumulated dose in study on cardiac transplantation patients3), and Fisher and Bickel reported that osteonecrosis shows a high association with daily dose, especially when doses exceed 20 mg per day in patients that have undergone renal transplantation9).
Kanter and Samuels reported about the anesthesia for major operations on patients who have transplanted heart. These patients are certainly at increased risk for anesthesia, but with proper management and careful monitoring using the standard patient anesthesia protocol, the risk of anesthesia can be reduced to reasonable leves10). In 1977 Burton et al. reported bilateral THA in two patients followed for 14 and 6 months respectively11). In 1986 Isono and Woolson evaluated 10 cardiac transplant recipients who have had bilateral total hip arhtroplasties (9 patients) and bilateral knee arthroplasties (1 patient). The follow-up of this patients revealed good range of motion of the operated joints without any report of complication12). Although the follow-up period was short in both studies, they conclude that the complication rate for THA in heart transplant recipients was similar to that in patients who did not have heart transplant.
Some have argued that cemented THA influences osteonecrosis of the femoral head in patients treated with adrenocortical hormones. In these patients, bone formation is suppressed and bone resorption is increased13). Accordingly, the authors advocated cemented THA based on concern that cementless THA could disturb endosteal new bone formation. After cementless THA, new endosteal bone formation on the porous coated surfaces of femoral stem is required for stability, although stable fibrous ingrowth also ensures satisfactory results. Thus, the most important factor is early stability. Furthermore, Engh et al. showed that reduced bone mass and quality per se do not influence new bone formation14). We performed cementless THA using the MIS-2-incision technique, which has the advantages of reducing bleeding during surgery and allowing an early return to normal activities and by minimizing injury to muscle15). In particular, in coronary transplant patients, which have a high risk of postoperative complications, this technique minimizes bed rest postoperatively and reduces systemic complications, such as, atelectasis and aspiration pneumonia.
Increased numbers of cardiac transplantations and improved patient survival rates are likely to cause a sustained increase in the number of cases of osteonecrosis of the femoral head.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download