Abstract
Background
Spinocerebellar ataxia (SCA) type 8 (SCA8) is an inherited neurodegenerative disorder caused by the expansion of untranslated CTA/CTG triplet repeats on 13q21. The phenomenology of SCA8 is relatively varied when compared to the other types of SCAs and its spectrum is not well established.
Case Report
Two newly detected cases of SCA8 with the nonataxic phenotype and unusual clinical manifestations such as dopaminergic-treatment-responsive parkinsonism and amyotrophic lateral sclerosis (ALS) are described herein. Family A expressed good dopaminergic treatment-responsive parkinsonism as an initial manifestation and developed mild cerebellar ataxia with additional movements, including dystonic gait and unusual oscillatory movement of the trunk, during the disease course. The proband of family B presented as probable ALS with cerebellar atrophy on brain MRI, with a positive family history (a brother with typical cerebellar ataxia) and genetic confirmation for SCA8.
Spinocerebellar ataxia (SCA) type 8 (SCA8) is an inherited neurodegenerative disorder caused by the expansion of untranslated CTA and CTG triplet repeats on 13q21. It was first described in a large American kindred (MN-A family) characterized by pure cerebellar ataxia similar to those of other SCAs with slow disease progression.1 The affected individuals in that family had longer repeat tracts than the 21 asymptomatic individuals. These results indicate that repeat length plays a role in disease penetrance.2 However, further analysis of other families showed that the pathogenic range can vary among families and that a positive test for repeat expansion, regardless of the repeat size, cannot be used to predict whether an asymptomatic individual will develop ataxia.3,4 Normal alleles have 15-50 repeats, while not all expanded alleles (>50 repeats) are pathogenic. Individuals with ataxia most often have 80-250 repeats; however, repeat sizes ranging from 71 to more than 1300 repeats have been found both in individuals who develop ataxia and those who do not. While it is now clear that CTA/CTG repeat expansion can cause ataxia, several issues have yet to be clarified, including reduced penetrance, gender effects, normal and pathogenic expansion ranges, detection in other neurodegenerative psychiatric disorders and even in the normal population.5-11
Furthermore, the phenomenology of SCA8 is relatively variable than those of the other types of SCA, and its spectrum variance is not well established.12 A large number of clinical analyses have demonstrated that SCA8 presents as a very slowly progressive ataxia,13,14 and affected individuals displayed gait, limb ataxia, speech, and oculomotor incoordination. However, there have been several reports of nonataxic symptoms of SCA8 as atypical phenotypes in the past decade. 15-18 Those reports suggest that there are molecular, genetic, and clinical heterogeneities in SCA8. Two newly detected cases with a nonataxic SCA8 phenotype are described herein, and the various nonataxic phenotypes and possible pathogenic mechanisms are discussed.
We identified two cases with SCA8 confirmed by genetic analysis. We obtained consents from the patients and performed neurological exams, and collected blood samples. The clinical features, disease course, family history, and co-morbidities were also investigated and analyzed in these two newly diagnosed cases. This study was approved by the Institutional Review Board of the Samsung Medical Center, Seoul, Korea. The pedigrees of the two cases are shown in Fig. 1A and B.
A 49-year-old, right-handed man (II: 3) (Fig. 1A) initially presented with a 6-year history of progressive gait disturbance. He had no history of drug exposure, trauma, or other medical problems, but his family history revealed a similar gait problem in his brother. The initial neurological examination revealed rigidity and bradykinesia on the right side, and a mildly decreased postural reflex [Unified Parkinson's Disease Rating Scale (UPDRS) part III score, 29; Hoehn and Yahr stage 2.5]. He did not have any behavioral or psychiatric problems, cognitive impairment (score on the Korean version of the Mini-Mental State Examination score of 30/30), or autonomic dysfunction. His parkinsonism responded well to a dopamine agonist and his condition improved considerable under that treatment (UPDRS part III score, 14; Hoehn and Yahr stage 2).
At the age of 51, mild cerebellar dysfunction including dysmetria and dysdiadochokinesia of both hands developed and additional movements such as dystonic posture of the right arm and leg during gait and unusual oscillatory up and down movement of the trunk, which occurred irrespective of medications, were observed. Brain MRI was performed to evaluate the unexpected, additional movement, and it revealed mild cerebellar atrophy (Fig. 1C). Molecular genetic analysis identified a combined 103 CTA/CTG expansion on 13q21. Genetic studies of other ataxia loci, including SCA1, SCA2, SCA3, SCA6, SCA7, dentatorubropallidoluysian atrophy (DRPLA), and Huntington's disease (HD) revealed no abnormalities. Positron-emission tomography using 18F-N-(3-fluoropropyl)-2β-carbonethoxy-3β-(4-iodophenyl) nortropane (FP-CIT) revealed reduced FP-CIT binding in the bilateral posterior putamen (Fig. 1D). During medical treatment the patient's disability had not progressed, his activities of daily living were not affected, and dysautonomia had not developed. The patient remained successfully treated with dopamine agonist monotherapy at the last follow-up (when aged 52 years).
The older brother of the proband (II: 1) (Fig. 1A) experienced dragging of his left leg at the age of 33 years and was consequently diagnosed with idiopathic Parkinson's disease (IPD) in another hospital. He showed good levodopa response, but his symptoms progressed slowly. A neurologic examination at our clinic at the age of 59 years revealed rigidity and bradykinesia in the left side, mild gait ataxia with widened base, and impaired tandem gait (UPDRS part III score, 21; Hoehn and Yahr stage 2.5). Additionally, unusual oscillatory movements of the neck and trunk from side to side were observed, which were not affected by medication. Although 26 years had passed since the onset of symptom, he showed favorable course without motor fluctuation, impairment of activities of daily living and autonomic dysfunction. He declined further genetic or imaging studies and was referred back to his prior hospital.
A 65-year-old, right-handed man (II: 5) (Fig. 1B) initially presented with a 5-year history of dysarthria. Dysarthria was slowly progressive and he became unintelligible at the age of 63, but he did not feel any other unwellness. However, dysphagia, dyspnea, generalized weakness, and weight loss (18 kg over 2 years) developed and progressed slowly during the 6 months before his initial visit to our clinic. He denied a history of drug or toxin exposure, trauma, or other medical problems, but had a family history of a deceased brother (II: 3) (Fig. 1B) who had previously been diagnosed with SCA8 with typical cerebellar ataxia by genetic sequencing at our clinic (combined 81 CTA/CTG expansion). He exhibited generalized muscle atrophy including tongue and intrinsic hand muscles. Neurological examination revealed flaccid-type dysarthria and muscle tone, facial diplegia, generalized muscle wasting and weakness (Medical Research Council grade 4), fasciculation in the tongue and thigh, and increased jaw and knee jerk without sensory disturbance and pathologic reflex. He showed unsteady gait, but it was difficult to determine whether unsteadiness was due to generalized weakness or cerebellar dysfunction. Video-oculography revealed vertical nystagmus during the head positional and shaking test. Nerve conduction test revealed no abnormal results, but electromyography revealed widespread denervation in the tongue, all levels of the trunk, and both the upper and lower extremities. Brain MRI revealed mild cerebellar atrophy (Fig. 1E). Genetic analysis identified a combined 86 CTA/CTG expansion on 13q21. Genetic studies for SCA1, SCA2, SCA3, SCA6, SCA7, DRPLA, superoxide dismutase for amyotrophic lateral sclerosis (ALS), and androgen receptor gene mutation for spinal and bulbar muscular atrophy revealed no abnormalities. Although he started to take riluzole, rapid progression of dysphagia and respiratory muscle weakness resulted in aspiration pneumonia and dyspnea.
The clinical presentation of SCA8-positive patients is characterized by cerebellar ataxia of varying severity, occurring alone or with other features. However, the recent accumulation of cases with atypical phenotype is making it apparent that the clinical presentation of SCA8 is variable, and no constellation of symptoms with predictive diagnostic value has been conclusively identified. There have been no reports of SCA8 patients in whom the disease mimics good dopaminergic treatment-responsive parkinsonism and ALS, and the clinical phenotypes of our cases are unusual compared with other reports of nonataxic phenotypes of SCA8.
In family A, II: 3 expressed asymmetric parkinsonian phenotype that included rigidity, bradykinesia and gait disturbance and showed good and sustained response to dopaminergic treatment initially. However, additional movement including dystonic gait, unusual oscillatory movement of trunk, and mild cerebellar ataxia developed later. He had a family history in his older brother (II: 1) who showed similar combined symptoms. The older brother (II: 1) in particular showed benign disease course for 26 years and it was not compatible with typical nature of IPD. Although both of these patients developed mild cerebellar ataxia later in the course of their disease, there was no evidence of multiple system atrophy (MSA), such as dysautonomia. Although atypical parkinsonism was reported as a phenomenology of SCA8, it was associated with a poor response to levodopa, with additional features implying corticobasal degeneration15 or MSA.6 Given the clinical profiles of the benign course and the family history of similar combined movements, dopaminergic treatment-responsive parkinsonism as an atypical phenotype of SCA8 is more plausible than an incidental detection or coexistence of CTG repeat expansion in these patients.
In family B, II: 5 presented as probable ALS with combined lower and upper motor neuron signs and mild cerebellar dysfunction. Motor neuron disease has been described as accompanied with SCA2,19 SCA620 and recently detected SCA36,21 but never with SCA8. This patient had cerebellar atrophy on brain MRI and exhibited vertical nystagmus during the head positional and shaking test, implying cerebellar dysfunction. Furthermore, his family history included his brother having typical cerebellar ataxia and genetic confirmation. Although the possibility of a rare coincidental association between SCA8 and sporadic motor neuron disease cannot be excluded, the clinical manifestation mimicking motor neuron disease has not previously been reported as an atypical phenotype of SCA8 to our knowledge.
The unusual symptomatology of SCA8 presenting as nonataxic phenotype has been described previously in the literature (Table 1). Furthermore, rare coexistence or incidental detection of the ataxin 8 gene repeat expansion has been observed in neurodegenerative disorders such as MSA, HD,16,22 and Alzheimer's disease,9 and in ataxias such as SCA1,8 SCA2,9 SCA6,23 16q-linked autosomal dominant cerebellar ataxia, and Friedreich's ataxia.
It is generally assumed that the cerebellum is the most vulnerable region of the brain in SCA disease states. The SCA8 transcript has been reported to down-regulate Kelch-like protein 1 (KLHL1) expression through an antisense mechanism, leading to SCA8 neuropathogenesis.24-28 Chen et al.26 demonstrated that the expression profile of KLHL1 overlaps with the brain areas affected in SCA8. Interestingly, expression of KLHL1 was widely distributed throughout the brain, mainly in the Purkinje cell layer of cerebellar cortex, but also highly distributed in motor-related regions including striatum, substantia nigra, pyramidal tract, spinal cord, and cognition-related areas including the hippocampus and lateral entorhinal cortex. These findings imply the existence of other potentially vulnerable regions that have yet to be characterized in SCA8 brains. It leads to the hypothesis that mutation of the SCA8 might affect neurons other than the cerebellum, and hence, the diverse phenotypes would be seen with SCA8. We demonstrated decreased uptake of FP-CIT in bilateral posterior putamen by imaging techniques and detected combined upper and lower motor neuron dysfunction in SCA8 patients. These support the nigrostriatal pathway and pyramidal tract as possible affected sites in SCA8.
In conclusion, the findings of our cases suggest that mutation of the SCA8 locus affect neurons other than the cerebellum and lead to non-ataxic phenotypes. Non-ataxic phenotype and its heterogeneity can make the diagnosis of SCA8 challenging and confuse clinicians when considering SCA8, and indicate the need for genetic testing in the appropriate context. We might consider SCA8 as a possible diagnosis in patients with complex non-ataxic clinical manifestation when family history is detected or cerebellar dysfunction develops later. The study of larger kindred and clues on the SCA8 biological function will help to better understand the pathogenic role of SCA8 alleles and the clinical diversity of the disease.
Figures and Tables
Fig. 1
A: Pedigrees of family A. B: Pedigrees of family B. Black boxes indicate the patients who express the clinical manifestations. Although II: 1 of family A revealed similar clinical phenotype with the proband (II: 3), he was not confirmed by genetic sequencing. C: Brain MRI of II: 3 (family A) revealed mild cerebellar atrophy. D: FP-CIT positron-emission tomography of II: 3 (family A) showed reduced FP-CIT binding in bilateral posterior putamen. E: Mild cerebellar atrophy was shown on brain MRI of II: 5 (family B).
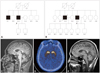
Table 1
Clinical features of SCA8 patients with non-ataxic phenomenology

AAE: age at evaluation, AAO: age at onset, AD: autosomal dominant, ALS: amyotrophic lateral sclerosis, ANS: autonomic dysfunction, AR: autosomal recessive, Ca: calcium, CBD: corticobasal degeneration, DTR: deep tendon reflex, HD: Huntington disease, IPD: idiopathic Parkinson disease, NA: not available.
References
1. Koob MD, Moseley ML, Schut LJ, Benzow KA, Bird TD, Day JW, et al. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat Genet. 1999; 21:379–384.

2. Day JW, Schut LJ, Moseley ML, Durand AC, Ranum LP. Spinocerebellar ataxia type 8: clinical features in a large family. Neurology. 2000; 55:649–657.

3. Ikeda Y, Dalton JC, Moseley ML, Gardner KL, Bird TD, Ashizawa T, et al. Spinocerebellar ataxia type 8: molecular genetic comparisons and haplotype analysis of 37 families with ataxia. Am J Hum Genet. 2004; 75:3–16.

4. Moseley ML, Schut LJ, Bird TD, Koob MD, Day JW, Ranum LP. SCA8 CTG repeat: en masse contractions in sperm and intergenerational sequence changes may play a role in reduced penetrance. Hum Mol Genet. 2000; 9:2125–2130.

5. Ikeda Y, Shizuka M, Watanabe M, Okamoto K, Shoji M. Molecular and clinical analyses of spinocerebellar ataxia type 8 in Japan. Neurology. 2000; 54:950–955.

6. Factor SA, Qian J, Lava NS, Hubbard JD, Payami H. False-positive SCA8 gene test in a patient with pathologically proven multiple system atrophy. Ann Neurol. 2005; 57:462–463.

7. Vincent JB, Yuan QP, Schalling M, Adolfsson R, Azevedo MH, Macedo A, et al. Long repeat tracts at SCA8 in major psychosis. Am J Med Genet. 2000; 96:873–876.
8. Sulek A, Hoffman-Zacharska D, Zdzienicka E, Zaremba J. SCA8 repeat expansion coexists with SCA1--not only with SCA6. Am J Hum Genet. 2003; 73:972–974.

9. Sobrido MJ, Cholfin JA, Perlman S, Pulst SM, Geschwind DH. SCA8 repeat expansions in ataxia: a controversial association. Neurology. 2001; 57:1310–1312.

10. Worth PF, Houlden H, Giunti P, Davis MB, Wood NW. Large, expanded repeats in SCA8 are not confined to patients with cerebellar ataxia. Nat Genet. 2000; 24:214–215.

11. Juvonen V, Hietala M, Päivärinta M, Rantamäki M, Hakamies L, Kaakkola S, et al. Clinical and genetic findings in Finnish ataxia patients with the spinocerebellar ataxia 8 repeat expansion. Ann Neurol. 2000; 48:354–361.

12. Gupta A, Jankovic J. Spinocerebellar ataxia 8: variable phenotype and unique pathogenesis. Parkinsonism Relat Disord. 2009; 15:621–626.

13. Brusco A, Cagnoli C, Franco A, Dragone E, Nardacchione A, Grosso E, et al. Analysis of SCA8 and SCA12 loci in 134 Italian ataxic patients negative for SCA1-3, 6 and 7 CAG expansions. J Neurol. 2002; 249:923–929.

14. Tazón B, Badenas C, Jiménez L, Muñoz E, Milà M. SCA8 in the Spanish population including one homozygous patient. Clin Genet. 2002; 62:404–409.

15. Baba Y, Uitti RJ, Farrer MJ, Wszolek ZK. Sporadic SCA8 mutation resembling corticobasal degeneration. Parkinsonism Relat Disord. 2005; 11:147–150.

16. Bereznai B, Lovas G, Pentelenyi K, Rudas G, Molnar MJ. Coexisting huntingtin and SCA8 repeat expansion: case report of a severe complex neurodegenerative syndrome. J Neurol Sci. 2010; 293:116–118.

17. Wu YR, Chen IC, Soong BW, Kao SH, Lee GC, Huang SY, et al. SCA8 repeat expansion: large CTA/CTG repeat alleles in neurological disorders and functional implications. Hum Genet. 2009; 125:437–444.

18. Wu YR, Lin HY, Chen CM, Gwinn-Hardy K, Ro LS, Wang YC, et al. Genetic testing in spinocerebellar ataxia in Taiwan: expansions of trinucleotide repeats in SCA8 and SCA17 are associated with typical Parkinson's disease. Clin Genet. 2004; 65:209–214.

19. Nanetti L, Fancellu R, Tomasello C, Gellera C, Pareyson D, Mariotti C. Rare association of motor neuron disease and spinocerebellar ataxia type 2 (SCA2): a new case and review of the literature. J Neurol. 2009; 256:1926–1928.

20. Ohara S, Tsuyuzaki J, Hayashi R, Iwahashi T, Nakajima T, Maruyama T, et al. Motor neuron loss in a patient with spinocerebellar ataxia type 6: chance co-occurrence or causally related? J Neurol. 2000; 247:386–388.

21. Kobayashi H, Abe K, Matsuura T, Ikeda Y, Hitomi T, Akechi Y, et al. Expansion of intronic GGCCTG hexanucleotide repeat in NOP56 causes SCA36, a type of spinocerebellar ataxia accompanied by motor neuron involvement. Am J Hum Genet. 2011; 89:121–130.

22. Koutsis G, Karadima G, Pandraud A, Sweeney MG, Paudel R, Houlden H, et al. Genetic screening of Greek patients with Huntington's disease phenocopies identifies an SCA8 expansion. J Neurol. 2012; 259:1874–1878.

23. Izumi Y, Maruyama H, Oda M, Morino H, Okada T, Ito H, et al. SCA8 repeat expansion: large CTA/CTG repeat alleles are more common in ataxic patients, including those with SCA6. Am J Hum Genet. 2003; 72:704–709.

24. Moseley ML, Zu T, Ikeda Y, Gao W, Mosemiller AK, Daughters RS, et al. Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat Genet. 2006; 38:758–769.

25. Ikeda Y, Daughters RS, Ranum LP. Bidirectional expression of the SCA8 expansion mutation: one mutation, two genes. Cerebellum. 2008; 7:150–158.

26. Chen WL, Lin JW, Huang HJ, Wang SM, Su MT, Lee-Chen GJ, et al. SCA8 mRNA expression suggests an antisense regulation of KLHL1 and correlates to SCA8 pathology. Brain Res. 2008; 1233:176–184.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download