Abstract
Background and Purpose
Galvanic vestibular stimulation (GVS) is a low-cost and safe examination for testing the vestibulospinal pathway. Human T-lymphotropic virus 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a slowly progressive disease that affects the vestibulospinal tract early in its course. This study compared the electromyographic (EMG) responses triggered by GVS of asymptomatic HTLV-1-infected subjects and subjects with HAM/TSP.
Methods
Bipolar galvanic stimuli (400 ms and 2 mA) were applied to the mastoid processes of 39 subjects (n=120 stimulations per subject, with 60 from each lower limb). Both the short latency (SL) and medium latency (ML) components of the EMG response were recorded from the soleus muscles of 13 healthy, HTLV-1-negative adults (56±5 years, mean±SD), and 26 individuals infected with HTLV-1, of whom 13 were asymptomatic (56±8 years) and 13 had HAM/TSP (60±6 years).
Results
The SL and ML EMG components were 55±4 and 112±10 ms, respectively, in the group of healthy subjects, 61±6 and 112±10 ms and in the HTLV-1-asymptomatic group, and 67±8 and 130±3 ms in the HAM/TSP group (p=0.001). The SL component was delayed in 4/13 (31%) of the examinations in the HTLV-1-asymptomatic group, while the ML component was normal in all of them. In the HAM/TSP group, the most common alteration was the absence of waves.
Vestibular-evoked myogenic potentials (VEMPs) are generated by a vestibulospinal reflex that can be triggered by acoustic or galvanic stimuli.1 The acoustic stimulus must be intense enough to generate a muscular response (e.g., clicks with amplitudes of 100 dB nHL), while galvanic vestibular stimulation (GVS) can vary in intensity and current duration, yet still generate similar and more robust responses.1,2
Galvanic vestibular stimulation is applied transcranially, and more commonly in a binaural configuration. The stimulus affects the firing rates of the irregular primary vestibular afferents, exciting on the negative (cathode) and inhibiting on the positive (anode) side of the electrodes.3,4 The changes in the vestibular input, through brainstem-spinal descending systems (mainly vestibulospinal tracts), exert a strong influence on body posture.5 Following GVS, the electromyographic (EMG) response is considered to have a spinal descending velocity comparable to that of the corticospinal tract.2 The discharges cause reciprocal changes in the activities of the trunk and limb muscles of both sides, resulting in a lateral body sway toward the anode, followed by a counteracting movement.6,7 The muscle responses to GVS are interpreted as a protective reflex to maintain postural control following an unexpected vestibular stimulus, which produces a corrective and unified response that passes through the descending spinal pathways.8 EMG responses have been recorded from the paraspinal, triceps brachii, tibialis anterior, and soleus muscles.2,9,10
The EMG response recorded over the soleus muscle produces a biphasic response, with the short-latency (SL) component initiating at approximately 60 ms after stimulus onset, followed by the medium-latency (ML) component, which initiates at around 100 ms after stimulus onset and in the opposite polarity to the SL.2,3,11 These responses are present only if the tested muscle is engaged in balance control, and are absent if the individual is seated,2 supporting the hypothesis that there is a task-dependent gating of descending vestibulospinal influences.7
The components of the soleus EMG response, although recorded consecutively, do not seem to be related to the same spinal pathway. When the pulse duration of the galvanic current was changed from 25 to 400 ms, a proportional increase in the ML component was observed, while there were no concurrent changes in the SL component.2 In another controlled experiment, the SL component was not affected when the somatosensory input was reduced through cooling of the skin on the sole of the foot, while there was an increase in the ML component.12 Moreover, providing visual information or finger support in order to increase proprioception did not change in the SL component but reduced the ML component.2 The same pattern of EMG response was found when the head was bent forward to diminish the dynamic effect under the semicircular canals.13 Ageing is the critical factor associated with a reduction in the amplitude of the SL component and an increase in the amplitude of the ML component; these changes are caused by the senescent decrease in the irregularly discharging primary afferents related to fast conduction.14 These findings suggest that the SL component of the soleus EMG response is a stable and direct measure of the vestibulospinal reflex,2,15 and that different vestibular end organs project via separate spinal pathways to reach their target motor neurons. The assumption is that the SL component of the response is driven from the otolith organs to the reticulospinal tract, whereas the ML component is driven from the semicircular canals to the vestibulospinal tract.13
Galvanic vestibular stimulation is a safe, low-cost, and easily reproducible examination that has been studied intensively by physiologists in experiments with both humans and other animals, which has increased knowledge of the entire vestibular system. Since this examination tests the neurophysiological integrity of vestibular-related descending tracts, we believe it may be useful for the diagnosis of nontraumatic myelopathies in their initial phase.
The model of spinal disease studied here was human T-cell lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP). There is epidemiological evidence for a causal association between HTLV-1 infection and HAM/TSP, and the geographic distribution of the virus is characterized by clusters of seropositivity, with its prevalence being highest in Japan, Africa, the Caribbean islands, and South America.16 This slowly progressive myelopathy predominates at the lateral column and at the ventral portion of the posterior column, particularly from the middle to lower thoracic levels.17 The diagnosis of HAM/TSP is generally late and currently based on clinical criteria and the detection of HTLV-1 antibodies in the serum or cerebrospinal fluid.18 Strategies for the early diagnosis of HAM/TSP have been widely studied because of epidemiological and future therapeutic implications. With a view to reducing the time to diagnosis, it has been demonstrated that triggering VEMPs by auditory stimulation is a valuable test for investigating cervical myelopathy in HAM/TSP.19
This study compared soleus EMG responses triggered by GVS in asymptomatic HTLV-1 carriers and HAM/TSP individuals, with the objective of testing the hypothesis that GVS may evoke an abnormal EMG response in apparently asymptomatic HTLV-1 carriers, whose response patterns may differ with the degree of neurological damage.
The Interdisciplinary Research Group on HTLV-1/2 [Global Institute of Public Health (GIPH)] is currently investigating the natural history, clinical manifestations, and epidemiological aspects of HTLV infection.20,21 This group has been following individuals infected with HTLV in the state of Minas Gerais, Brazil, in an open cohort since 1997, with over 570 HTLV-infected and noninfected (healthy control group) individuals presently being followed for more than 10 years.20 The seroprevalence of HTLV-1 infection among former blood donors in Minas Gerais is 0.32%.22
For this sectional study, a total of 39 participants from the GIPH cohort submitted to GVS, and their EMG responses were recorded from the soleus muscle of each lower limb. The participants had no previous history of either middle-ear or vestibular disease and were not taking any vestibular suppressant medication at the time of the study. The control group comprised 13 HTLV-1-negative individuals aged 56±5 years (mean±SD). The group infected with HTLV-1 comprised 26 individuals, who were divided into two subgroups: 13 were clinically asymptomatic and aged 56±8 years, and 13 had a definite diagnosis of HAM/TSP and were aged 60±6 years. The subjects with HAM/TSP were all able to remain standing without assistance (standing is essential for recording VEMPs from the lower limbs).2,3
This research was conducted in accordance with Resolution 196/96 of the National Health Council, and was approved by the Federal University of Minas Gerais Ethics Committee, under review no. ETIC 0437.0.203.000-10. All subjects provided written informed consent to participate.
Galvanic vestibular stimulation comprised direct, monophasic, and rectangular current pulses with an amplitude and duration of 2 mA and 400 ms, respectively (model EvP4/ATCPlus, Contronic, Pelotas, Brazil). The stimuli were delivered at random intervals of 4-5 s, and responses to a total of 120 stimulations were averaged. The bipolar current was applied to the mastoid bones using 3-cm-diameter self-adhesive surface electrodes (model CF3200, ValuTrode, Axelgaard, Fallbrook, CA, USA). Both of the following current stimulation polarities were used for transmastoid binaural stimulation: cathode left/anode right (CLAR), and cathode right/anode left (CRAL). The stimulus polarity was computer-controlled and randomized across trials. The earth electrode was placed over the sternum. GVS was divided into four blocks of 30 stimuli each, with 30 responses recorded from the left lower limb (15 CLAR stimuli and 15 CRAL stimuli) and 30 from the right lower limb (15 CLAR stimuli and 15 CRAL stimuli). To ensure repeatability, the procedure was repeated consecutively in all participants, totaling 60 stimulations from each lower limb.
During the examination, the subjects remained standing in bare feet on a flat surface with their eyes closed, feet close together, and body slightly bent forward so as to contract the soleus muscle. Subjects were instructed to rotate their head at approximately 90° from the sagittal plane, contralateral to the lower limb from which the EMG signals were to be measured, because responses are stronger and more consistent in the lower limb contralateral to the direction of head rotation.23
Electromyographic activity was measured using self-adhesive electrodes (model MediTrace 300, Kendall, USA). Each pair of recording electrodes was placed vertically at 2 cm below the popliteal fossa and separated by approximately 1 cm. The reference electrode was attached to the back of the thigh approximately 3 cm above the upper recording electrode (Fig. 1). The electrodes were moved from one lower limb to the other after each 30-stimulus block. A sufficient rest period between blocks was provided to prevent muscle fatigue.
The EMG signals were averaged, rectified, band-pass filtered between 10 Hz and 1 kHz, and digitized at a sampling frequency of 5 kHz. The data were collected during successive 500-ms periods, starting 100 ms before the galvanic stimulus. The responses were observed in real time during each examination (Fig. 2).
The analysis of EMG responses was based on the lower limb with a contralateral head rotation. Averaged traces for the two electrode-placement combinations were superimposed after digital filtering and subtraction of the prestimulus rectified EMG traces. The responses that inverted their polarity in the face of stimulation under both polarity conditions (i.e., CRAL and CLAR) were considered to be vestibular in origin.2,3 The latencies of the two soleus response components related to the vestibular-evoked reflex (SL and ML) were examined visually and measured using the cursor. The SL component was considered as any response elicited between 40 and 70 ms after stimulus onset that had an inverted trace and a reversing stimulus polarity; the polarity of the ML component was opposite that of the SL component, and it occurred about 100 ms after stimulus onset.2,3 The first divergence on the traces was marked as the onset of the SL component. In each sequence the traces returned to baseline and then diverged again. The second divergence on the traces marked the onset of the ML component. The end of the response was defined as the point at which the traces finally returned to the baseline level (Fig. 2). The averages of the two sets of replicated responses for each participant were calculated to determine single values for the SL and ML components.
The dependent variables were the onsets of the SL and ML components, and wave presence or absence.14 An abnormal response was identified when at least one of the following conditions was observed: 1) a delayed latency relative to the values observed for the control group of healthy subjects (with a deviation exceeding mean±2SD) or 2) wave absence based on an undetectable response (Fig. 3). The analysis was carried out by two separate examiners who were blind to the HTLV-1 statuses of the subjects. Amplitude was not considered in this study since it is influenced by age and muscular strength and so could have acted as a confounding factor in the analysis.14
Statistical analyses were performed using the Statistical Package for the Social Sciences for Windows version 18.0 (SPSS Inc., Chicago, IL, USA). ANOVAs with contrasts were performed using SL and ML components as dependent variables and HTLV-1 groups as fixed factors. The Bonferroni correction was used to account for the multiple comparisons tests. Significance was set at p<0.05. Between-group comparisons for categorical variables were conducted using the Pearson chi-square test (or the Fisher test, when small samples were involved).
The 13 HTLV-1-negative healthy subjects were aged 56±5 years, the 13 asymptomatic HTLV-1 carriers were aged 56±8 years, and the 13 HAM/TSP subjects were aged 60±6 years. All three study groups comprised three men and ten women.
The EMG response had the same shape for all participants in the control group, with SL and ML components of 55±4 and 112±10 ms, respectively (Fig. 2). The SL and ML components were 61±6 and 112±10 ms, respectively, in the asymptomatic HTVL-1 group, and 67±8 and 130±3 ms in the HAM/TSP group (Fig. 3). The latency of the EMG SL component was prolonged in the subjects with asymptomatic infection, whereas in the subjects with HAM/TSP the pattern varied from a prolonged latency of both components to complete absence of an evoked response (Fig. 4).
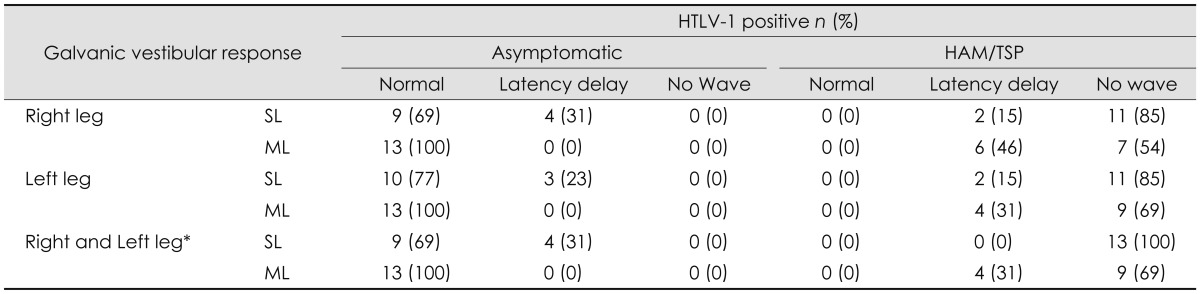
The frequency of occurrence of an abnormal lower-limb SL responses in at least one of the legs was 4/13 (31%) among the HTLV-1-asymptomatic carriers and 13/13 (100%) among the HAM/TSP subjects. Conversely, the ML component was normal in the asymptomatic group and altered in the HAM/TSP group, in which the most common finding was the absence of a response. Table 1 lists the types of alteration of the EMG response observed in this study.
Comparison of EMG responses between HTLV-1 asymptomatic carriers and HAM/TSP subjects revealed that they varied along a spectrum from normal to anomalous. The alteration was first observed in the SL component, followed by the ML component, and ultimately in a complete absence of an evoked response.
This study evaluated VEMPs generated by galvanic stimulation in individuals infected with HTLV-1, with or without myelopathy, and analyzed the electrophysiological aspects of the vestibulospinal pathway. The vestibulospinal tract has not been routinely assessed in clinical practice, although balance disorders are being more common due to many factors, including overall population ageing. The vestibulospinal tract forms part of the descending spinal pathways, along with the reticulospinal and corticospinal tracts.5,7 Hashimoto et al.24 studied the integrity of the corticospinal tract in individuals with HAM/TSP using evoked motor potentials elicited by transcranial magnetic stimulation. Despite consistent results showing delayed motor responses, which could improve the diagnosis of HAM/TSP and help predict the progression and outcome of the disease, the high cost of this method of stimulation limits its applications in clinical practice.
A model of neurological disease with individuals who were at different clinical phases (asymptomatic and with HAM/TSP) was used in the present study to investigate alterations in the EMG response. These alterations ranged from prolonged latencies to undetectable responses, which may indicate that the damage to the vestibulospinal tract in this disease is progressive.
The findings of two studies that used GVS to evaluate traumatic spinal cord injury were consistent with a response latency delay in minor trauma and an undetectable response in major trauma, and corroborate the present results.10,15 Liechti et al.15 evaluated soleus EMG responses in six individuals with incomplete spinal cord injury due to trauma and concluded that the prolonged ML response latency was caused by lesions in the vestibulospinal pathway.
The independent but correlated responses of the two soleus EMG response components is supported by the results of experiments that analyzed the factors that change the latency or the shape of each component separately.2,12-14 The first component (i.e., SL) represents the mechanisms of fast conduction associated with the reaction of the balance system evoked by a pure vestibular perturbation, while the second component (i.e., ML) is more elaborate and polysynaptic, and is susceptible to changes in the external environment.7 It seems that HTLV-1-related neurological damage affects the SL-component pathway first. Interestingly, the inflammatory process that characterizes this neurological disease starts at the large myelinated fibers of the corticospinal tract, which are related to the fast conduction velocities associated with the SL component.7,17,25
A small proportion (4%) of asymptomatic carriers will eventually develop HAM/TSP, which has prompted immunological and clinical studies aimed at identifying a prognosis biomarker.26 Dizziness is a frequent complaint in HAM/TSP when compared with the clinical features of other nontraumatic myelopathies, and reportedly appears earlier than other common symptoms such as bladder disorders.25 The morphological alterations caused by HAM/TSP suggest explanations for the cause of dizziness.17,25 HAM/TSP is an inflammatory myelopathy that is almost restricted to the descending spinal pathways.25 The damage to the reticulospinal and vestibulospinal tracts, both of which are associated with balance control, may explain the high frequency of dizziness in HAM/TSP subjects.27 Subclinical alterations can occur among the asymptomatic HTLV-1-infected population.17,18 Therefore, the EMG abnormalities detected in subjects who appear to be asymptomatic may be used as a sign to predict HAM/TSP. Vestibular-evoked EMG responses that differ according to the progression of neurological disease, from asymptomatic carriers to patients complaining of walking difficulty, and finally to patients with definite HAM/TSP. Felipe et al.19 tested the cervical spine of HTLV-1-infected individuals using VEMPs triggered by intense acoustic stimuli, and two parameters were considered for comparison: latency prolongation and lack of an EMG response. Delayed latency was detected in 50% of the apparently asymptomatic carriers, while lack of an EMG response predominated in 80% of individuals with HAM/TSP. The present study used galvanic stimuli instead of acoustic stimuli; the advantage of the former is that it reaches the lumbar spine, whereas the latter triggers responses of the vestibulospinal tract only up to the second cervical spinal nerve. In accordance with the results of Felipe et al.,19 in the present study, latency prolongation predominated in asymptomatic carriers, whereas the absence of an EMG response predominated in HAM/TSP subjects. These findings support the presence of a correlation between neural conduction and the type of EMG alteration. Severe and massive neural lesions usually lead to total conduction failure with no EMG response, known as conduction block, and delayed evoked responses have traditionally been attributed to impaired focal conduction.28
To our knowledge, GVS has not previously been considered for evaluating nontraumatic myelopathies. The physiopathology of HAM/TSP is associated with an inflammatory network, but unlike other infectious diseases such as schistosomal myeloradiculopathy, for which the treatment is curative,29 no effective treatment is available yet for HAM/TSP.25 Schistosomal myeloradiculopathy is endemic in many developing countries, including some areas of Brazil, and similarly to HAM/TSP the damage predominates at the thoracic and lumbar spinal cord level.25,29 The prognosis is better upon early treatment, and relapse is challenging because it can lead to worse sequelae.29,30 The results of the present study show that GVS can uncover subclinical HTLV-1 disease or relapse, and its implementation in clinical practice may therefore improve monitoring of the functional outcome of this disease.
In conclusion, GVS is useful for detecting spinal cord lesions, since 100% of the subjects with HAM/TSP in this study had altered EMG responses to GVS, and these alterations in EMG response were observed even at an apparently asymptomatic stage of the infection. The accuracy of this diagnostic tool may be better determined by examining the VEMP patterns of individuals with asymptomatic HTLV-1 infection, and their risk of developing HAM/TSP.
Acknowledgements
The authors thank audiologist Lilian Felipe and engineers Gustavo Ott and Vinicius Torchelsen Alves for their contribution on galvanic vestibular stimulation.
This work was financially supported by National Council for Scientific and Technological Development (CNPq) and PRPQ.
References
1. Watson SR, Colebatch JG. Vestibular-evoked electromyographic responses in soleus: a comparison between click and galvanic stimulation. Exp Brain Res. 1998; 119:504–510. PMID: 9588785.

2. Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993; 94:143–151. PMID: 8335069.

3. Fitzpatrick R, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol. 1994; 478(Pt 2):363–372. PMID: 7965852.

4. Goldberg JM, Smith CE, Fernández C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984; 51:1236–1256. PMID: 6737029.

5. Muto N, Shinomiya K, Komori H, Mochida K, Furuya K. Spinal cord monitoring of the ventral funiculus function Analysis of spinal field potentials after galvanic vestibular stimulation. Spine (Phila Pa 1976). 1995; 20:2429–2434. discussion 2435. PMID: 8578394.
6. Séverac Cauquil A, Martinez P, Ouaknine M, Tardy-Gervet MF. Orientation of the body response to galvanic stimulation as a function of the inter-vestibular imbalance. Exp Brain Res. 2000; 133:501–505. PMID: 10985684.

7. Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. 2004; 96:2301–2316. PMID: 15133017.

8. Day BL, Séverac Cauquil A, Bartolomei L, Pastor MA, Lyon IN. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol. 1997; 500(Pt 3):661–672. PMID: 9161984.

9. Baldissera F, Cavallari P, Tassone G. Effects of transmastoid electrical stimulation on the triceps brachii EMG in man. Neuroreport. 1990; 1:191–193. PMID: 2129879.

10. Iles JF, Ali AS, Savic G. Vestibular-evoked muscle responses in patients with spinal cord injury. Brain. 2004; 127(Pt 7):1584–1592. PMID: 15128616.

11. Watson SR, Colebatch JG. EMG responses in the soleus muscles evoked by unipolar galvanic vestibular stimulation. Electroencephalogr Clin Neurophysiol. 1997; 105:476–483. PMID: 9448650.

12. Muise SB, Lam CK, Bent LR. Reduced input from foot sole skin through cooling differentially modulates the short latency and medium latency vestibular reflex responses to galvanic vestibular stimulation. Exp Brain Res. 2012; 218:63–71. PMID: 22278107.

13. Cathers I, Day BL, Fitzpatrick RC. Otolith and canal reflexes in human standing. J Physiol. 2005; 563(Pt 1):229–234. PMID: 15618274.

14. Welgampola MS, Colebatch JG. Selective effects of ageing on vestibular-dependent lower limb responses following galvanic stimulation. Clin Neurophysiol. 2002; 113:528–534. PMID: 11955997.

15. Liechti M, Müller R, Lam T, Curt A. Vestibulospinal responses in motor incomplete spinal cord injury. Clin Neurophysiol. 2008; 119:2804–2812. PMID: 18842452.

16. Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005; 24:6058–6068. PMID: 16155612.

17. Izumo S. Neuropathology of HTLV-1-associated myelopathy (HAM/TSP). Neuropathology. 2010; [Epub ahead of print].

18. De Castro-Costa CM, Araújo AQ, Barreto MM, Takayanagui OM, Sohler MP, da Silva EL, et al. Proposal for diagnostic criteria of tropical spastic paraparesis/HTLV-I-associated myelopathy (TSP/HAM). AIDS Res Hum Retroviruses. 2006; 22:931–935. PMID: 17067261.
19. Felipe L, Gonçalves DU, Santos MA, Proietti FA, Ribas JG, Carneiro-Proietti AB, et al. Vestibular-evoked myogenic potential (VEMP) to evaluate cervical myelopathy in human T-cell lymphotropic virus type I infection. Spine (Phila Pa 1976). 2008; 33:1180–1184. PMID: 18469690.

20. Allain JP, Stramer SL, Carneiro-Proietti AB, Martins ML, Lopes da Silva SN, Ribeiro M, et al. Transfusion-transmitted infectious diseases. Biologicals. 2009; 37:71–77. PMID: 19231236.

21. Gonçalves DU, Proietti FA, Ribas JG, Araújo MG, Pinheiro SR, Guedes AC, et al. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev. 2010; 23:577–589. PMID: 20610824.

22. Proietti FA, Lima-Martins MV, Passos VM, Brener S, Carneiro-Proietti AB. HTLV-I/II seropositivity among eligible blood donors from Minas Gerais State, Brasil. Vox Sang. 1994; 67:77. PMID: 7975458.

23. Lund S, Broberg C. Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand. 1983; 117:307–309. PMID: 6603098.

24. Hashimoto T, Uozumi T, Tsuji S. Paraspinal motor evoked potentials by magnetic stimulation of the motor cortex. Neurology. 2000; 55:885–888. PMID: 10994018.

25. Goncalves DU, Proietti FA, Barbosa-Stancioli EF, Martins ML, Ribas JG, Martins-Filho OA, et al. HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) inflammatory network. Inflamm Allergy Drug Targets. 2008; 7:98–107. PMID: 18691139.

26. Kaplan JE, Osame M, Kubota H, Igata A, Nishitani H, Maeda Y, et al. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J Acquir Immune Defic Syndr. 1990; 3:1096–1101. PMID: 2213510.
27. Peterson BW, Abzug C. Properties of projections from vestibular nuclei to medial reticular formation in the cat. J Neurophysiol. 1975; 38:1421–1435. PMID: 1221080.

28. Murofushi T, Shimizu K, Takegoshi H, Cheng PW. Diagnostic value of prolonged latencies in the vestibular evoked myogenic potential. Arch Otolaryngol Head Neck Surg. 2001; 127:1069–1072. PMID: 11556854.

29. Drummond SC, Pereira SR, Silva LC, Antunes CM, Lambertucci JR. Schistosomiasis control program in the state of Minas Gerais in Brazil. Mem Inst Oswaldo Cruz. 2010; 105:519–523. PMID: 20721502.

30. Felipe L, Gonçalves DU, Tavares MC, Sousa-Pereira SR, Antunes CM, Lambertucci JR. Vestibular-evoked myogenic potential (VEMP) in the evaluation of schistosomal myeloradiculopathy. Am J Trop Med Hyg. 2009; 81:551–554. PMID: 19815864.

Fig. 1
Procedure for eliciting the soleus EMG response under GVS. The active electrodes were placed vertically on the skin surface around the soleus muscle at 2 cm below the popliteal fossa and separated by approximately 1 cm. The reference electrode was attached to the back of the thigh approximately 3 cm above the upper recording electrode. EMG: electromyographic, GVS: galvanic vestibular stimulation.

Fig. 2
Signals captured from the right soleus muscle. The thick line indicates the response from the anode placed over the right mastoid process, and the thin line is the response from the anode on the left side. The SL arrow indicates the onset of the short-latency response; the ML arrow indicates the end of the short-latency response and the onset of the medium-latency response. ML: medium latency, SL: short latency.

Fig. 3
The EMG responses followed a pattern of alteration that started with an increase in the latency of the SL component followed by an increase in the latency of the ML component. The latencies were longer for the HAM/TSP group than for the HTLV-1-asymptomatic and control groups. p=significance probability (t-test). HTLV-1: Human T-lymphotropic virus 1, ML: medium latency, SL: short latency.
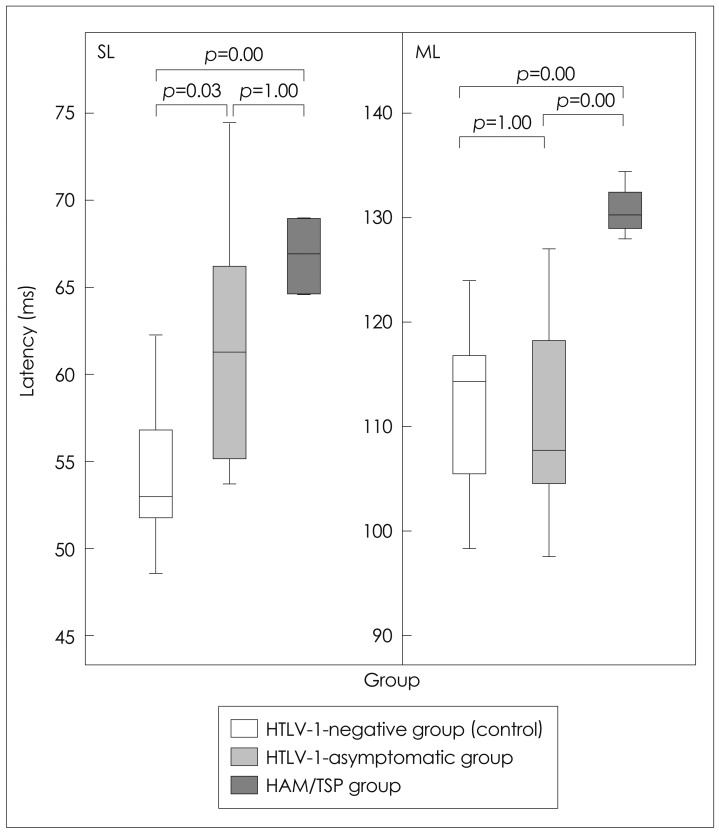
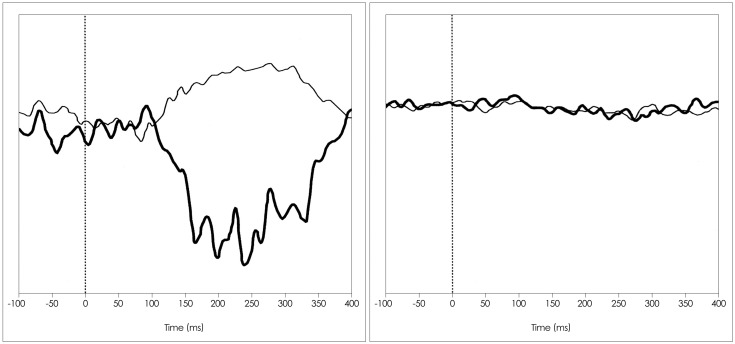
Fig. 4
The upper trace shows the delayed response of the SL component, which was the most common alteration observed in the HTLV-1-asymptomatic group. The lower trace shows the absence of a response, which was the most common alteration observed in the HAM/TSP group. HAM/TSP: HTLV-1 associated myelopathy/tropical spastic paraparesis, HTLV-1: Human T-lymphotropic virus 1, SL: short latency.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download