Abstract
Background and Purpose
Intracranial atherosclerotic stenosis (ICAS) is considered as a major cause of stroke. The carotid intima-media thickness (CIMT), which accurately reflects the burden of generalized atherosclerosis, is also associated with stroke. The aim of this study was to determine the association between the CIMT and ICAS responses to medical treatment.
Methods
This study constituted part of the "Trial of cilostazol in symptomatic intracranial arterial stenosis"-2 that evaluated the ICAS response after randomized antiplatelet treatment. Magnetic resonance angiography and CIMT measurement were performed at baseline and after 7 months of treatment. CIMT was measured using semiautomated software, and was presented as maximum (CIMT-max) and average (CIMT-ave) values. The change in CIMT was compared relative to the ICAS response (i.e., progression, no-change, and regression). Ordinal logistic regression and analysis of covariance (ANCOVA) were used to analyze the association between the responses.
Results
Among the 101 enrolled patients, 85 underwent follow-up CIMT measurement. CIMT increased most in the ICAS progression group (CIMT-max: 0.09±0.23, CIMT-ave: 0.04±0.12), and to a lesser degree in the no-change group (CIMT-max: 0.02±0.16, CIMT-ave: 0.02±0.11), but decreased in patients with ICAS regression (CIMT-max: -0.04±0.11, CIMT-ave: -0.03±0.07; CIMT-max: p=0.010, CIMT-ave: p=0.015). Ordinal logistic regression analysis demonstrated that the change in CIMT-max was independently associated with the ICAS response (p=0.032). However, the ANCOVA revealed that the reverse was not true, in that the ICAS response was not independently associated with the change in CIMT after adjusting for confounding factors.
Intracranial atherosclerotic stenosis (ICAS) is one of the major causes of ischemic stroke. Although the progression of ICAS is a strong predictor for stroke recurrence,1 the nature and risk factors of ICAS progression have yet to be clarified. In particular, the association between the natural course of ICAS and systemic atherosclerosis has never been investigated.
Carotid intima-media thickness (CIMT) is a well-established marker of generalized atherosclerosis. Its relationship with atherosclerosis in arteries at various sites has been reported previously. Increased CIMT was associated with the presence and extent of atherosclerosis in the coronary arteries,2 peripheral arteries,3 and the carotid bifurcation or internal carotid artery.4 A recent community-based cross-sectional study performed in an Asian country found that CIMT was increased in subjects with ICAS. However, the increased CIMT was not independently associated with ICAS after adjusting for confounding factors.5
There are pathophysiologic differences in the occurrence and development of atherosclerosis between the intra- and extracranial arteries, and these differences may have influenced the previous results.5 However, the correlation between the responses of atherosclerosis to medical treatment in the intraand extracranial arteries has not yet been elucidated. Therefore, the present study investigated the change in CIMT relative to the degree of ICAS response to medical treatment in ischemic stroke patients with symptomatic ICAS.
This study was performed as a substudy of the "Trial of cilostazol in symptomatic intracranial arterial stenosis" (TOSS)-2 (unique identifier: NCT00130039),6 which is a randomized controlled multicenter trial that enrolled 457 acute (≤2 weeks of onset) ischemic stroke patients with symptomatic ICAS in the middle cerebral or basilar arteries, as assessed by magnetic resonance angiography (MRA). To ensure that only patients with intracranial stenosis due to atherosclerosis were included, patients with 1) other vasculopathy such as arterial dissection or moyamoya disease, 2) embolic heart disease, or 3) significant stenosis of arteries proximal to the symptomatic stenosis were excluded. After obtaining their written informed consent to participate, subjects who were enrolled in TOSS-2 were randomly assigned to either the cilostazol (100-mg aspirin plus 200-mg cilostazol daily) or clopidogrel (100-mg aspirin plus 75-mg clopidogrel daily) groups. Aggressive control of atherosclerosis risk factors including statin therapy was strongly recommended in the protocol. The progression of ICAS between the two antiplatelet regimens was compared after 7 months. The TOSS-2 study is described in detail elsewhere.6
In the present substudy, demographic characteristics and clinical data including vascular risk factors were obtained at baseline. The results of various blood tests including lipid profiles, fasting glucose, and C-reactive protein (CRP) were also obtained. Three-dimensional time-of-flight MRA and carotid ultrasonography were used to evaluate the baseline ICAS and CIMT, respectively. After 7 months of treatment, the details of any concomitant medications used during the observation period were obtained, and the blood tests, MRA, and carotid ultrasonography were followed-up so that the responses to treatment could be evaluated. The protocol was approved by the ethics committee of each participating center.
MRA was performed twice: first at baseline and then at the 7 months follow-up. According to the TOSS-2 MRA grading system, the severity of ICAS was classified into normal (0), mild (1; signal reduction <50%), moderate (2; signal reduction >50%), severe (3; focal signal loss with distal flow), or occlusion (4; sudden cutoff without distal flow void). The change in ICAS after 7 months of treatment was categorized into three groups: progression, regression, and no-change. The severity of symptomatic ICAS was evaluated independently by two investigators who were blinded to all of the clinical information. If any discrepancies arose, a third investigator resolved the discrepancy by consensus with the other two investigators.
Six of the 20 centers that participated in TOSS-2 were capable of assessing the CIMT in acute ischemic stroke patients, and could therefore participate in this substudy. Before the data acquisition began, a sample carotid ultrasonography image was assessed from each center to evaluate the appropriateness of image quality. Carotid ultrasonography was performed according to a standard protocol by skilled sonographers at each center, and using the same schedule as for MRA. CIMT was measured from each right and left carotid artery with a 10-MHz linear vascular probe. The insonation angle was 120-150° for the left side and 210-240° for the right side; the variation among patients meant that the best angle for measurement was set by the examiner. The depth of focus was 30-40 mm and the log gain for measurement was approximately 60 dB. Standardized longitudinal B-mode images were obtained from the far wall of the common carotid artery, which was defined as the segment extending from 10 to 20 mm proximal to the tip of the bifurcation site according to the Mannheim carotid intima-media thickness consensus.7 All of the DICOM images that contained the proper CIMT for each side were archived electronically from each center to the central laboratory for CIMT measurements.
Carotid intima-media thickness was measured using dedicated semiautomated software (Intimascope, Media Cross, Tokyo, Japan) by a single reader (S.R.K.) who was blinded to all clinical information to avoid interrater disagreement.8 The resulting data are presented as the maximum (CIMT-max) and average (CIMT-ave) values. CIMT-max was calculated as the mean of the maximum points in both common carotid arteries, while CIMT-ave was the mean of the average value obtained from 250 computer-based points for each common carotid artery.8
General characteristics including demographic characteristics, vascular risk factors, and concomitant medications were compared relative to the ICAS response to treatment. Changes in lipid profiles, fasting glucose, CRP, and CIMT after 7 months of medical treatment were also compared. The ICAS response was used as a categorical variable with an ordinal nature. The analysis was performed using chi-square statistics for a linearby-linear association model and appropriate contrast weights from ANOVA. Bonferroni's post-hoc analysis was also performed in order to compare the change in CIMT between the progression and regression groups.
Ordinal logistic regression models were used to test the presence of an independent association between the change in CIMT and the ICAS response after adjusting for potential confounding factors. All of the variables that exhibited a potential association (i.e., p<0.15) with the ICAS response in bivariate analysis were selected. Any suspicion of multicollinearity between CIMT-max and CIMT-ave was prevented by using two models. Models 1 and 2 both contained sex, smoking, and the type of antiplatelet agent, with model 1 containing CIMT-max whereas, model 2 containing CIMT-ave. In addition, a potential independent association between the ICAS response and the follow-up CIMT-max or CIMT-ave was evaluated using separate models of analysis of covariance (ANCOVA) after adjusting for age, sex, hypertension, diabetes, hyperlipidemia, smoking, type of antiplatelet, and baseline CIMT. Statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Except where stated otherwise, the data are presented as mean±SD values, and the level of statistical significance was set at p<0.05.
Among the 457 patients who were enrolled into TOSS-2 from the 20 centers, 223 patients were registered from the 6 centers that were able to offer carotid ultrasonography. This substudy was proposed after the initiation of TOSS-2; therefore, the 122 patients who had already been registered to TOSS-2 before the commencement of this substudy could not receive carotid ultrasonography and were unable to participate in this substudy. Among the remaining 101 patients, follow-up carotid ultrasonography was not performed in 16 patients due to various causes, and hence 85 patients were ultimately included in the present study. Due to technical problems, ultrasonography was performed on one side only in two patients. ICAS progressed in 13 (15.3%) patients, regressed in 29 (34.1%) patients, and remained unchanged in 43 (50.6%) patients.
There were no significant differences among the groups with respect to demographic characteristics, risk factors, and concomitant medications relative to the ICAS response (Table 1), nor in lipid profiles, fasting glucose, and CRP between baseline and follow-up (Table 2). However, there was a significant change in CIMT according to the relative progression of ICAS. The CIMT increased in patients with ICAS progression (CIMT-max: 0.09±0.23 mm and CIMT-ave: 0.04±0.12 mm) or with no change in ICAS status (CIMT-max: 0.02±0.16 mm and CIMT-ave: 0.02±0.11 mm). Conversely, CIMT decreased in patients with ICAS regression (CIMT-max: -0.04±0.11 mm and CIMT-ave: -0.03±0.07 mm). The trend analysis demonstrated a significant linear decrease in CIMT among those with a favorable ICAS response to treatment (CIMT-max: p=0.010; CIMT-ave: p=0.015) (Fig. 1).
Post-hoc analysis between patients with ICAS regression and progression revealed that the change in CIMT-max decreased significantly in the ICAS regression group (mean difference= 0.14; p=0.037). The results of ordinal logistic regression demonstrated that the change in CIMT-max was independently associated with the ICAS response after adjusting for potential confounding factors (p=0.032) (Table 3). However, the ICAS response was not independently associated with the follow-up CIMT after adjusting for age, sex, conventional risk factors, type of antiplatelet, and baseline CIMT (CIMT-max, ANCOVA F=2.677, ANCOVA p=0.075; CIMT-ave, ANCOVA F=1.508, ANCOVA p=0.228).
The objective of this study was to determine the association between the ICAS and CIMT responses to medical treatment. The results indicated that CIMT decreased in patients with ICAS regression and increased both in patients with no change in ICAS status and in those with ICAS progression. This illustrates a similarity in the CIMT and ICAS responses. The change in CIMT-max differed significantly between the ICAS progression and regression groups, and was also independently associated with the ICAS response. However, there was no statistically significant independent association between the ICAS response and the follow-up CIMT after adjusting for confounding factors.
Various factors may influence the occurrence and progression of atherosclerosis in the intra- and extracranial arteries. ICAS is more closely associated with hypertension, diabetes, and metabolic syndrome than is atherosclerosis in the carotid arteries.9 Patients with ICAS exhibited higher CIMTs, whereas the association disappeared after adjusting for risk factors from a previous study.5 Focusing on the progression of atherosclerosis, systemic endothelial dysfunction was associated with the progression of CIMT,10 whereas low high-density lipoprotein cholesterol levels were associated with symptomatic ICAS progression.11 Although the risk factors of atherosclerosis occurrence and progression differ according to the site, the present results demonstrate that the responses of atherosclerosis in distinct sites may actually be associated with each other.
Increased CIMT is frequently observed in ICAS patients,12 and medical treatment usually focuses on the target site. However, effective antiatherosclerotic treatments at one site may also be effective for other vascular beds. It has been shown previously that lipid-lowering agents prevent the progression of symptomatic ICAS,13 and are significantly associated with a favorable decrease in CIMT.14 Cilostazol, an antiplatelet agent with an antiatherogenic effect, has been shown to prevent the progression of both ICAS6 and CIMT.15
Only subjects with symptomatic ICAS participated in the present study. The change in CIMT-max was independently associated with the ICAS response. Therefore, during the medical treatment for ICAS, subjects exhibiting a favorable CIMT-max response may also exhibit a favorable ICAS response. However, the converse does not appear to be true, in that ICAS change was not associated with the follow-up CIMT (as indicated by ANCOVA). Therefore, subjects with a favorable ICAS outcome may not always demonstrate a favorable CIMT response. However, the small sample may have been at least partially responsible for the failure to demonstrate an independent association in both directions. Moreover, the results should be interpreted with further caution since the main trial included symptomatic ICAS patients and its focus was the treatment of ICAS and its response.
This substudy of TOSS-2 was subject to some limitations, the first of which stems from its small sample. Since the participation of each center was determined according to the feasibility, and the substudy was proposed during the main trial, the number of subjects available with a baseline CIMT was small. Second, volumetric evaluation of the change in plaque size was not included as an endpoint, and the measurement of plaque size by ultrasonography in multiple centers may have increased differences between the centers. Third, the measurement of CIMT may also vary considerably with the ultrasound setting, the study site, and the angle at which it is assessed.16 To minimize the possible effects of these factors, CIMT was measured according to a fixed protocol based on the Mannheim carotid intima-media thickness consensus,7 and the data were analyzed using semiautomated software. Although there were some intercenter differences in the baseline and follow-up CIMT values, the change in CIMT, which was the main outcome of this substudy, did not differ significantly among the centers.
In conclusion, there is a correlation between the ICAS response and the CIMT response to treatment in symptomatic ICAS patients. Therefore, the CIMT response, which is a surrogate marker of generalized atherosclerosis, may reflect the ICAS response to treatment.
Figures and Tables
Fig. 1
Difference in CIMT change relative to the ICAS response after treatment of symptomatic ICAS. CIMT: carotid intima-media thickness, ICAS: intracranial atherosclerotic stenosis.

Table 2
Difference in lipid profiles and changes in CIMT after antiplatelet therapy according to the ICAS response
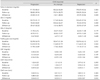
Table 3
Independent factors associated with the ICAS response

Variables entered to each model of ordinal logistic regression: Model 1: sex, smoking, type of antiplatelet agent, and CIMT-max, Model 2: sex, smoking, type of antiplatelet agent, and CIMT-ave.
CI: confidential interval, CIMT: carotid intima-media thickness, ICAS: intracranial atherosclerotic stenosis, SE: standard error.
Acknowledgements
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare Republic of Korea (HI10C2020).
Korea Otsuka Pharmaceutical, Korea Otsuka International Asia, and Korea Otsuka International Arab provided financial support for TOSS-2 and the CIMT substudy. However, these institutions played no role in the protocol development, data collection, or data analysis for the current study, or in the manuscript preparation.
References
1. Arenillas JF, Molina CA, Montaner J, Abilleira S, González-Sánchez MA, Alvarez-Sabín J. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke. 2001; 32:2898–2904.

2. Coskun U, Yildiz A, Esen OB, Baskurt M, Cakar MA, Kilickesmez KO, et al. Relationship between carotid intima-media thickness and coronary angiographic findings: a prospective study. Cardiovasc Ultrasound. 2009; 7:59.

3. Allan PL, Mowbray PI, Lee AJ, Fowkes FG. Relationship between carotid intima-media thickness and symptomatic and asymptomatic peripheral arterial disease. The Edinburgh Artery Study. Stroke. 1997; 28:348–353.

4. Bots ML, Hofman A, De Jong PT, Grobbee DE. Common carotid intima-media thickness as an indicator of atherosclerosis at other sites of the carotid artery. The Rotterdam Study. Ann Epidemiol. 1996; 6:147–153.

5. Leng XY, Chen XY, Chook P, Xiong L, Lin WH, Liu JY, et al. Correlation of large artery intracranial occlusive disease with carotid intima-media thickness and presence of carotid plaque. Stroke. 2013; 44:68–72.

6. Kwon SU, Hong KS, Kang DW, Park JM, Lee JH, Cho YJ, et al. Efficacy and safety of combination antiplatelet therapies in patients with symptomatic intracranial atherosclerotic stenosis. Stroke. 2011; 42:2883–2890.

7. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012; 34:290–296.

8. Yanase T, Nasu S, Mukuta Y, Shimizu Y, Nishihara T, Okabe T, et al. Evaluation of a new carotid intima-media thickness measurement by B-mode ultrasonography using an innovative measurement software, intimascope. Am J Hypertens. 2006; 19:1206–1212.

9. Bang OY, Kim JW, Lee JH, Lee MA, Lee PH, Joo IS, et al. Association of the metabolic syndrome with intracranial atherosclerotic stroke. Neurology. 2005; 65:296–298.

10. Halcox JP, Donald AE, Ellins E, Witte DR, Shipley MJ, Brunner EJ, et al. Endothelial function predicts progression of carotid intima-media thickness. Circulation. 2009; 119:1005–1012.

11. Kim DE, Kim JY, Jeong SW, Cho YJ, Park JM, Lee JH, et al. Association between changes in lipid profiles and progression of symptomatic intracranial atherosclerotic stenosis: a prospective multicenter study. Stroke. 2012; 43:1824–1830.

12. Jung KW, Shon YM, Yang DW, Kim BS, Cho AH. Coexisting carotid atherosclerosis in patients with intracranial small- or large-vessel disease. J Clin Neurol. 2012; 8:104–108.

13. Kim HJ, Kim EK, Kwon SU, Kim JS, Kang DW. Effect of statin on progression of symptomatic intracranial atherosclerosis. Can J Neurol Sci. 2012; 39:801–806.

14. Huang Y, Li W, Dong L, Li R, Wu Y. Effect of statin therapy on the progression of common carotid artery intima-media thickness: an updated systematic review and meta-analysis of randomized controlled trials. J Atheroscler Thromb. 2013; 20:108–121.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download