Abstract
Background
Myasthenia gravis (MG) and myotonic dystrophy type 2 (DM2) are rare disorders individually, and their coexistence in the same patient is very rare. We present a patient in which these two diseases coexisted.
Case Report
The patient complained of diplopia, fluctuating limb weakness, and difficulties in swallowing and speaking. A neurological examination revealed diplopia, facial, weakness of the neck and proximal limb muscles, dysphagia, dysphonia, and myotonia. The patient's mother had DM2 and her maternal grandfather had cataracts. MG was confirmed in our patient by positive results for neostigmine and a repetitive nerve stimulation test, and elevated serum anti-acetylcholine-receptor antibodies, while DM2 was confirmed by electromyography and genetic testing. The patient improved remarkably after treatment with anticholinesterases, corticosteroids, and azathioprine.
Conclusions
This is the second reported case of the coexistence of DM2 and MG in the same patient. Since the symptoms of these two diseases overlap it is very important to keep in mind the possibility of their coexistence, so that MG is not overlooked in patients with a family history of myotonic dystrophy.
Myasthenia gravis (MG) is an autoimmune disease characterized by muscle weakness and fatiguability due to postsynaptic impairment of neuromuscular transmission. Myotonic dystrophy type 2 (DM2) is characterized by myotonia and muscle weakness, pain, and stiffness, and less commonly by cardiac conduction defects, cataracts, type 2 diabetes mellitus, and gonadal failure. Expansion of the CCTG repeat in the CNBP (ZNF9) gene on chromosome 3 causes DM2.
We report the coexistence of these two disorders in a single patient.
A 30-year-old woman first noticed double vision 2 years prior to hospitalization. It had been present periodically, mostly in the evenings, and did not disturb the patient sufficiently to seek medical attention. Three months later she noticed leg weakness with walking difficulties, especially when climbing and walking down stairs, and walking instability resulting in consecutive falls. Six months later weakness of the arms appeared that caused difficulties in washing and combing her hair. After seven months she noticed difficulties in swallowing and nasal speech after prolonged talking. All of these symptoms had a fluctuating course and were more pronounced in the evenings.
A neurological examination revealed diplopia due to the weakness and fatiguability of the rectus superior and rectus lateralis muscles of the left eye as well as of the rectus inferior of the right eye, moderate weakness of the facial muscles, mild dysphagia with nasal speech, mild weakness of neck muscles, and weakness and fatiguability of proximal limb muscles. Very mild active and percussion myotonia were observed, but the patients had not been aware of it prior to hospitalization. The patient's mother had been diagnosed with DM2 at the age of 48 years and her maternal grandfather had cataracts.
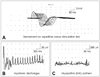
A diagnosis of MG was confirmed in our patient by clear positive results in the neostigmine test and decremental responses in the repetitive nerve stimulation test (RNST) (Fig. 1A); these were 43% and 26% in the deltoid and nasal muscles, respectively. Anti-acetylcholine receptor (AChR) antibody titer was elevated (8.8 nmol/L, normal <0.2 nmol/L). A chest CT scan showed a persistent thymic tissue in the upper anterior mediastinum, and a pathohistological examination of the thymus after thymectomy revealed thymic hyperplasia. Electromyography during relaxation demonstrated diffuse episodes of myotonic discharges (Fig. 1B) in multiple muscles, while during voluntary muscle activity a mild myopathic lesion was revealed by short-duration motor-unit action potentials and early recruitment (Fig. 1C). Genetic testing confirmed the presence of DM2. The patient improved remarkably after treatment with anticholinesterases, corticosteroids, and azathioprine.
It is very unusual for MG and DM2-which are individually two rare disorders-to be diagnosed in a single patient. Only seven patients with coexistence of MG and myotonic dystrophy have been reported previously.1-7 The coexistence of MG and myotonic dystrophy type 1 was confirmed in three of these patients,2,5,6 the authors did not report the results of genetic testing in another three of them,1,3,4 while MG and DM2 coexisted in the seventh case.7
The coexistence of these two conditions could be difficult to ascertain without a high level of suspicion and specific laboratory testing. Some of the symptoms of these two diseases overlap, so one disease can easily be missed, especially MG in a patient with a known family history of myotonic dystrophy. Muscle fatigue is the most prominent symptom in MG, and this is also one of the most disturbing symptoms of DM2.8
In our patient the diagnosis of MG was suspected due to the presence of fluctuating weakness of proximal limb muscles, double vision, and difficulties in swallowing and speaking. Proximal muscle weakness is also characteristic of DM2, but its fluctuating character was more consistent with MG. Also, double vision and bulbar muscle weakness do not form part of the DM2 clinical presentation. The diagnosis of MG was confirmed by positive neostigmine test results, decremental responses in the RNST, elevated anti-AChR antibody titer, and persistent thymic tissue in the mediastinum. The diagnosis of myotonic dystrophy was suspected due to the presence of myotonia and a positive family history of myotonic dystrophy, and this was confirmed by Electromyography and genetic testing. Cataract, cardiac conduction defects, hypogammaglobulinemia, insulin insensitivity, and primary gonadal failure were not detected in our patient.
We are aware that nonspecific positive results can be obtained in the neostigmine test in several other conditions, including muscle dystrophies and motor neuron disease. Also, the RNST can show increased decremental responses in different myopathies, including myotonic dystrophy type 1. On the other hand, the RNST results are mostly normal in DM2, so we attributed the positive results in the RNST in our patient to impairment of myasthenic postsynaptic neuromuscular transmission. On the other hand, an elevated anti-AC hR antibody titer is highly specific for MG and has never been reported previously in a patient with myotonic dystrophy.
Our patient responded very well to treatment with anticholinesterases, corticosteroids, and azathioprine. Although anticholinesterase agents have been reported to aggravate myotonia in myotonic dystrophy and myotonia congenita,9 our patient responded well to pyridostigmine with no side effects.
To the best of our knowledge, this is only the second reported case of the coexistence of DM2 and MG in the same patient. Although very rare, the possibility of this coexistence should be kept in mind, so that MG-which is easily treatable but at the same time a very serious disease if untreated-is not overlooked in patients with a family history of myotonic dystrophy.
Figures and Tables
References
1. Schoen RT. Myasthenia gravis and myotonic dystrophy in a 13-year-old girl. G. Milton Shy Award Essay, 1976. Neurology. 1977. 27:546–549.

2. de los Angeles Avaria M, Kleinsteuber K, Novoa F, Faundez P, Carvallo P. Myotonic dystrophy in a female with myasthenia gravis. Pediatr Neurol. 2007. 36:421–423.

3. Maytal J, Spiro AJ, Sinnar S, Moshe SL. The coexistence of myasthenia gravis and myotonic dystrophy in one family. Neuropediatrics. 1987. 18:8–10.

4. Karagoz ET, Midi I, Tanridag T. Coexistence of myasthenia gravis and myotonic dystrophy in a thyrotoxicosis patient. Internet J Neurol. 2008. 9:8.
5. Feyma T, Carter GT, Weiss MD. Myotonic dystrophy type 1 coexisting with myasthenia gravis and thymoma. Muscle Nerve. 2008. 38:916–920.

6. Benito-León J, Porta-Etessam J, Díaz de Bustamante A. Myasthemia gravis and myotonic dystrophy in the same patient. Rev Neurol. 2001. 32:498.
7. Bamberg C, Deschauer M, Tews DS, Claus D. Coincidence of myasthenia gravis and myotonic dystrophy type 2. Klin Neurophysiol. 2008. 39:267–270.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download