Abstract
Background and Purpose
The aims of this study were to elucidate the cognitive functions of narcoleptics and determine whether intelligence protects against cognitive dysfunction and depressive mood in these patients.
Methods
Sixty-six subjects (33 narcoleptics, 33 controls) were administered a battery of neuropsychological tests and an individual standardized intelligence test. The cognitive functions of the narcoleptic patients and the healthy controls were compared, as were those of high-IQ and mid-to-low-IQ narcoleptic patients.
Results
Narcoleptics exhibited significantly lower scores in the Corsi Block-Tapping Test forward and backward, and the digit symbol tests, and significantly higher Beck Depression Inventory scores than the controls. However, verbal attention, verbal-visual long-term memory, and executive function task scores did not differ significantly between patients and controls. The mid-to-low-IQ patient group had lower mean digit span backward test, phonemic and semantic fluency Controlled Oral Word Association Test and Korean version of the Boston Naming Test scores, and a higher total score and general depressive symptoms subscales Beck Depression Inventory score than the high-IQ patient group. However, controls exhibited no IQ-related differences in cognitive performance or depressive mood. Patients in the high-IQ group exhibited impaired visual attention and working memory as compared with controls.
Conclusions
The findings of the present study show that narcolepsy patients have deficits in visual attention and visual working memory, and tend to feel more general depressive symptoms but not somatic symptoms than their control, nonnarcoleptic counterparts. In addition, it appears that higher intelligence protects against cognitive dysfunction and depressive mood.
Narcolepsy is a neurological disorder that is characterized by excessive daytime sleepiness or sudden sleep attacks with cataplexy, sleep paralysis, hypnagogic hallucinations, and fragmented nocturnal sleep.1-6 Early studies of cognitive impairment in narcoleptics were based mainly on self-reported complaints of patients or anecdotal clinical observations,7 and thus were unsuitable for exploring cognitive deficits in an objective manner. However, several of those studies did not reveal significant memory impairment in narcolepsy patients compared to normal controls, although some patients complained that they had increased difficulty in maintaining attention.8-10 On the other hand, other studies found that narcoleptics had selective cognitive deficits in response latency and word recall,11 or impaired abilities regarding attention and executive function tasks that require higher levels of inhibition or task management abilities.12 The inconsistencies of objective cognitive evaluations contrast with consistent patient complaints of declines in cognitive functions related to attention, concentration, executive function, and processing speed.9-12
Previous studies have suggested that intellectual function acts as a protective factor in terms of the recovery, adaptation, and psychological state of patients with psychiatric and neurological disorders.13-15 Although IQ in narcoleptics has been assessed previously using a computerized neurocognitive function tool and the Wechsler Intelligence Scale for Children (3rd edition),16,17 no previous study has investigated the effect of intelligence on cognitive functions in narcoleptics.
The aims of the present study were therefore to characterize a comprehensive range of cognitive functions in narcoleptic patients and determine whether significant differences in memory and concentration exist between narcoleptics and age-, gender-, education-, and IQ-matched healthy controls. In addition, we investigated whether intelligence protects against cognitive dysfunction and depressive mood in narcoleptics.
All of the narcoleptic subjects were selected at the sleep disorder clinic of a university hospital between June 2005 and October 2009. Thirty-three drug-naïve narcolepsy patients (21 men, 12 women) who met the International Classification of Sleep Disorders (2nd edition), and 33 age-, education-, and intelligence-matched healthy controls (19 men, 14 women) were recruited, the latter via a public notice at the hospital. Informed consent was obtained from all patients prior to its commencement, and the institutional review board of our hospital authorized the study protocol. The narcolepsy patients underwent history-taking and various sleep tests with overnight polysomnography (PSG) and the Multiple Sleep Latency Test (MSLT). In particular, we documented excessive daytime sleepiness, current cataplexy symptoms, cataplexy history, and the expansive quality of sleep through history-taking and the Epworth Sleep Scale (ESS). The exclusion criteria included 1) a sleep disorder causing excessive daytime sleepiness, such as sleep apnea, 2) a psychiatric or other neurological disorder, 3) a medical disorder that could cause sleep problems, 4) a history of head trauma, epilepsy, encephalitis, brain tumor, brain surgery, other relevant brain disorders, drug abuse or alcohol intoxication, a psychiatric disorder, impaired corrected visual or hearing ability or hand motor functions, or 5) an IQ of <80 according to the Korean version of the Wechsler Intelligence Scale (KWIS). The healthy controls had no history of neurological disease, notable cognitive dysfunction, psychiatric or medical disorder, or history of alcohol or drug abuse.
All 66 study subjects were administered a battery of neuropsychological tests and an individual standardized intelligence test. We administered the Doppelt form of the KWIS.18 The KWIS Doppelt form addresses the domains of vocabulary, mathematics, picture arrangement, and block design. To evaluate verbal memory, we administered the Korean version of the California Verbal Test.19 Visual memory was examined using the Rey Complex Figure Test.20 The Korean version of the California Verbal Test involves 5 learning steps and 16 words; after the 5 learning steps, we examined immediate recall and immediate hint recall, and 20 minutes later we examined delayed free recall, delayed hint recall, and recognition. The Rey Complex Figure Test involves immediate recall after copying a complex figure, and free recall and recognition 20 minutes later. The digit span test from the Wechsler Memory Scale-Revised was administered to evaluate verbal attention and working memory, using a standard protocol.21 The Corsi Block-Tapping Test (CBTT) was used to examine visual attention and working memory.22 We also administered Trail-Making Tests-A and -B and the digit symbol test.23
The emotional state of the subjects was examined used the Beck Depression Inventory (BDI), which comprises 21 items with 4 factors: cognitive, affective, motivational, and somatic.24 We focused on the BDI scores of the two scales to establish whether somatic symptoms prevail in narcoleptic patients: the general depressive symptoms and somatic symptoms subscales (impaired sleep, somatic symptoms, fatigue, loss of appetite, weight loss, and loss of libido).25,26 The following cognitive domains tests were also administered: 1) for executive functions, the Wisconsin Card-Sorting Test,27 the Stroop test,28 the Controlled Oral Word Association Test (COWAT),29 and Raven's Colored Progressive Matrices;30 and 2) for verbal functions, the Korean version of the Boston Naming Test (KBNT).31
Narcoleptic subjects submitted to an overnight PSG and MSLT in the sleep laboratory. The PSG was administered from 10 PM to 7 AM the following day, and the MSLT was commenced 2 hours after awakening. The MSLT comprised five naps scheduled at 2-hour intervals starting at 9 AM Patients were invited to lie down on a bed in a dark, soundproof room and instructed to try to fall asleep. Sleep latency was defined as the time elapsed from the start of the test (lights out) to the first 30-second epoch scored as sleep. Each sleep latency test was terminated 20 minutes after the onset of sleep or after 20 minutes of wakefulness. A sleep-onset REM period (SOREMP) was defined as one or more epochs of REM sleep occurring within 15 minutes of the first 30-second epoch scored as sleep.6
The independent t-test was used to evaluate between-group differences, while the χ2 test was used to evaluate differences associated with gender. Because of the small number of subgroups, the Mann-Whitney U test was used to evaluate subgroup differences between narcoleptics and controls. The data are presented as mean±SD values, and the level of statistical significance was set at p<0.05 (two-tailed). Statistical analyses were conducted using SPSS version 12.0 for Windows.
No significant differences were observed between patients and controls with respect to age (29.9±12.7 years vs. 30.1±12.7 years, p=0.94), gender (men: n=21 vs. 19; p=0.61), educational level (13.8±3.0 years vs. 13.4±1.5 years, p=0.43), or IQ (112.5±11.5 vs. 111.4±9.7, p=0.67). In narcoleptic patients, the age at onset of excessive daytime sleepiness was 14.8±7.8 years and the disease duration was 16.4±11.4 years. They had an ESS score of 14.5±3.7. Twenty-one patients (13 men, 9 women) had cataplexy, with an age at onset of 18.0±4.4 years. During MSLT, the sleep latency was 130.8±121.8 seconds, and two or more SOREMPs were observed in all patients.
Narcoleptic patients were stratified according to their IQ into a high-IQ group (IQ≥110, n=16) and a mid-to-low-IQ group (IQ≤109, n=17), and the characteristics of these groups were compared to determine any effect of IQ. No significant intergroup difference was observed with respect to age (32.8±12.6 years vs. 27.2±12.5 years, p=0.21), age at onset of excessive daytime sleepiness (14.9±8.1 years vs. 14.7±7.7 years, p=0.90), ESS score (14.5±3.4 vs. 14.6±4.1, p=0.84), or mean sleep latency during the MSLT (163.1±123.7 seconds vs. 100.4±115.3 seconds, p=0.06). However, mid-to-low-IQ patients had a significantly a longer disease duration (20.3±11.5 years vs. 12.7±10.2 years, p=0.02), a higher number of SOREMPs during the MSLT (3.9±1.1 vs. 2.9±1.1, p=0.02), and fewer educational years (12.4±2.6 years vs. 15.3±2.7 years, p=0.00).
Verbal memory did not differ significantly between the narcolepsy and control groups for the domains of total learning quantity, short-delay free recall, long-delay free recall, or recognition. Similarly, there were no significant differences between the two groups in visual memory for the copy, immediate recall, delayed recall, and recognition domains (Table 1). The scores for the digit span forward and backward tests also did not differ significantly. However, narcolepsy patients had a significantly lower CBTT-forward/-backward and digit symbol test scores (Table 2).
Narcoleptic patients reported significantly more depressed mood than controls, as assessed using the BDI (BDI score: 9.3±6.3 vs. 5.9±4.9, p=0.02), and experienced more general depressive symptoms (7.8±5.2 vs. 5.0±4.0, p=0.04), but the somatic symptoms of the BDI subscale did not differ significantly between the two groups (2.5±1.9 vs. 2.0±1.7, p=0.34). Furthermore, executive function and verbal function test scores did not differ significantly between narcoleptics and controls (Wisconsin Card-Sorting Test: 5.7±0.8 vs. 5.6±1.2, p=0.70; Raven's Colored Progressive Matrices: 34.6±1.7 vs. 34.4±1.6, p=0.55; Stroop test: 107.2±9.4 vs. 108.8±7.3, p=0.43; K-BNT: 52.8±4.4 vs. 52.4±5.3, p=0.78; COWAT-phonemic: 35.3±10.5 vs. 35.8±9.8, p=0.85; COWAT-semantic: 32.8±7.6 vs. 35.0±7.1, p=0.24).
Based on an IQ evaluation conducted using the KWIS, the narcolepsy patients were subdivided into those with a high score (IQ≥110, n=16) and those with a mid-to-low score (IQ≤109, n=17).18 The mid-to-low-IQ patient group performed significantly worse in the digit span backward test (7.7±1.8 vs. 9.5±1.8, p=0.01), the phonemic test (30.6±9.4 vs. 40.3±9.6, p=0.01), in semantic fluency as assessed using COWAT (30.0±6.3 vs. 35.9±7.9, p=0.03), and the K-BNT (50.6±4.5 vs. 55.1±2.4, p=0.00). They also had a higher BDI total score (11.8±5.9 vs. 6.8±5.8, p=0.02; Fig. 1) and a higher general depressive symptoms subscales score (10.1±4.8 vs. 5.7±4.9, p=0.01) than the high-IQ patient group. Furthermore, the mid-to-low-IQ patient group exhibited a tendency toward feeling more somatic symptoms (3.3±2.0 vs. 1.8±1.7, p=0.04).
As with the narcoleptic patients, the control subjects were divided into those with a high IQ score (IQ≥110, n=18) and those with a mid-to-low IQ score (IQ≤109, n=15).18 No significant differences were observed between these two groups with respect to age (25.9±10.0 years vs. 33.6±13.9 years, p=0.08), gender (men: n=13 vs. 6, p=0.06), or educational level (12.8±1.3 years vs. 13.8±1.6 years, p=0.06). Similarly, cognitive performance and BDI scores were did not differ significantly between them (Table 3).
Members of the high-IQ patient group (n=16) had spent significantly more years in higher education (15.3±2.7 years vs. 13.4±1.5 years, p=0.002) and had a higher IQ (121.6±9.1 vs. 111.4±9.7, p=0.001) than members of the controls, but performed worse in the digit symbol test (63.2±10.6 vs. 71.4±13.0, p=0.021). On the other hand, members of the mid-to-low-IQ patient group (n=17) had a significantly lower IQ (103.9±4.8 vs. 111.4±9.7, p=0.012), and lower scores in the CBTT-forward (8.2±1.9 vs. 9.9±2.3, p=0.014) and CBTT-backward (8.1±1.2 vs. 9.3±1.5, p=0.006) tests, the digit symbol test (62.5±13.9 vs. 71.4±13.0, p=0.021), and semantic fluency as assessed using COWAT (30.0±6.3 vs. 35.0±7.1, p=0.018). Furthermore, they were more depressed (as assessed using the BDI) than the control group (BDI score: 11.8±6.0 vs. 6.0±4.9, p=0.001) and felt more general depressive symptoms (BDI subscale score: 10.1±4.9 vs. 5.0±4.0, p=0.003). However, their somatic symptoms did not differ significantly from those of the controls (BDI subscale score: 3.3±2.0 vs. 2.0±1.8, p=0.56).
The ESS scores of narcolepsy patients were negatively correlated with Trail-Making Test-B scores (r=-0.35). There were no significant differences in general cognitive domains and depressive level between severely drowsy patients (ESS>15, n=16) and moderately drowsy patients (ESS≤15, n=17), or in cognitive domain scores between narcoleptics with a mean sleep latency of <3 min (n=25) and those with a mean sleep latency of >3 min (n=8).6
This study explored the cognitive and emotional characteristics of narcolepsy patients. Specifically, we investigated whether narcoleptics exhibit any objective decline in attention or memory as compared with gender-, age-, and IQ-matched normal controls. No significant differences were found between patients and controls in terms of verbal working or long-term memory, visual long-term memory, or auditory attention. However, narcoleptics exhibited significantly impaired temporary/sustained attention to a visual stimulus and visual working memory performance. Furthermore, we found that narcolepsy patients were more depressed than controls.
The effect of level of intelligence on cognitive functions and task performances was examined by stratifying both the narcoleptics and controls into high- and mid-to-low-IQ groups. The high-IQ patient group performed better than the mid-to-low-IQ patient group in terms of auditory attention and verbal fluency, and the latter had a significantly more depressive mood. On the other hand, there were no significant differences in cognitive performance between the high- and mid-to-low-IQ control groups. Furthermore, the narcolepsy patients were found to have impaired visual working memory and short-term attention performance compared to the controls, regardless of IQ. Moreover, there were no differences in cognitive performance between narcolepsy patients stratified according to their level of drowsiness (i.e., severely or moderately drowsy, as determined using ESS and MSLT scores).
It has been reported that narcolepsy patients experience a sense of anxiety and have low self-confidence regarding the execution of memory tasks, which may explain the common complaints of subjective memory decline.9 In the present study, the narcoleptics demonstrated a more depressed state than the controls. Contrary to expectations, the level of somatic symptoms did not differ significantly between narcoleptic patients and controls. Furthermore, since depression can negatively affect overall cognitive performance,33,34 we hypothesize that a sense of depression contributes to the perception of diminished memory function and a subjective feeling of memory and attention decline.
The narcoleptic patients included in this study scored statistically significantly lower in the CBTT and the digit symbol test, both of which require the processing of visual stimuli, demonstrating that narcoleptics cannot efficiently pay attention to visual stimuli and working memory, and that their attention capacities are limited. This finding is consistent with the subjective decline in attention reported by narcoleptics, because they take longer to perceive and inspect visual stimuli than do controls, resulting in additional difficulty when they perform these visual tasks. However, other studies have found contradictory results on this topic. For example, two studies found no difference between narcoleptics and normal controls in terms of visual attention, divided attention to a visual stimulus, and working memory.12,15 These conflicting results may be related to differences in the nature of cognitive tasks, and may be open to diverse interpretations.
Significant auditory attention impairment was not detected in the patients included in the present study, which concurs with the findings of some other studies.8,10,12 However, although yet another study found no difference between patients and controls with regard to attention tasks that required about 5 minutes, a significantly inferior performance was observed in narcoleptics in a different task that required longer than 10 minutes.35 In the memorization task in the present study, the external stimuli presented by an evaluator changed rapidly. During such a test, patients may monitor their awareness and spare their cognitive resources to adapt to changes in awareness, and therefore do not demonstrate a decline in attention. However, when patients are forced to perform tasks over longer periods of time without external stimuli from an evaluator, they may not be able to monitor awareness levels and use cognitive resources, which could result in a significant attention impairment. In the present study it was difficult to draw any conclusions in this regard since we did not use tests that took longer than 10 minutes to complete. Therefore, we recommend that future research employs additional long-duration tasks with diverse stimuli to allow a more complete evaluation of attention impairment.
Many previous studies have concluded that intelligence acts as a protective factor in terms of cognitive, social, and emotional aspects in those with a neurological or psychiatric disability.36,37 However, no study has previously investigated whether intelligence plays the same role in narcolepsy patients. Nevertheless, one study found that narcolepsy patients with a high IQ exhibited significant cognitive impairment, which suggests that intelligence and cognitive impairment are independent.15 When we compared cognitive function and emotional state in patients with different IQ levels in narcoleptics, we found that the high-IQ group performed better in terms of cognitive function and felt less general depressive symptoms. Although many different definitions exist, Wechsler defined intelligence as "aggregate or global capacity encompassing cognitive, emotional, and characteristic aspects, that is, the ability of an individual to act purposefully, to think rationally, and to deal effectively with his environment"37. Thus, intelligence describes the potential of an individual to achieve efficient adaptation, or it could be interpreted as a resource that can be used to compensate for the impairment in a certain factor, since intelligence is the combined result of multifaceted, multidetermined factors. This could be comprehended as cognitive reserve, which could be defined as an ability to engage alternative brain networks to compensate for neurological pathology and behavioral impairment.38-40 It has been demonstrated that a high cognitive reserve is a protective factor in schizophrenia, bipolar disorder, depression, and traumatic brain injury.38,39 From the perspective of task execution, intelligence can positively affect retrieval and the application of previous learning, and can play a protective role to compensate for impaired attention and memory.37,41 This may explain our finding that narcoleptics with a high IQ experience less cognitive impairment and are less depressed. Accordingly, we believe that the present study is of value because it probes whether the level of intelligence-an index of cognitive reserve-influences cognitive function and emotional state.
This study was subject to several limitations that should be considered. First, the subjective declines in memory and attention investigated in previous research studies were not investigated herein, and thus it was not possible to compare patient-evaluated subjective indices with objective indices derived from standardized tests. Second, only a shortened intelligence test was used to measure IQ. According to a study of patients with schizophrenia, the level of consistency between the Doppelt or WARD7 form intelligence tests and the overall level of consistency was 66-90%, which implies a limitation of the shortened test to closely examine an individual's intelligence.42 Third, we did not undertake an extensive evaluation of the emotional states of the narcolepsy patients; we therefore recommend that future studies compare subjective and objective indicators of memory function and use comprehensive testing to evaluate emotions.
Figures and Tables
 | Fig. 1Comparison of cognition and depressive mood between high-IQ and mid-to-low-IQ narcoleptic patients. The high-IQ group performed significantly better on visual attention and verbal fluency and felt a lower depressive mood than those with a mid-to-low IQ. BDI: Beck Depression Inventory, COWAT: Controlled Oral Word Association Test, K-BNT: Korean version of the Boston Naming Test. |
Table 3
Comparison of the cognitive performances and Beck Depression Inventory (BDI) score between high-IQ and mid-to-low-IQ controls
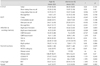
BNT: Boston Naming Test, CBTT: Corsi Block-Tapping Test, COWAT-P: Controlled Oral Word Association Test-Phonemic, COWAT-S: COWAT-Semantic, K-CVLT: Korean version of the California Verbal Learning Tests, RCFT: Rey Complex Figure Test, RCPM: Raven's Color Progressive Matricies, TMT: Trail-Making Test, WCST: Wisconsin Card Sorting Test.
Acknowledgements
This study was supported by a Grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (No. A110097) and by the Global Frontier R&D Program on <Human-centered Interaction for Coexistence> funded by the National Research Foundation of Korea grant funded by the Korean Government (MEST) (2012M3A6A3056079).
References
1. Draganski B, Geisler P, Hajak G, Schuierer G, Bogdahn U, Winkler J, et al. Hypothalamic gray matter changes in narcoleptic patients. Nat Med. 2002. 8:1186–1188.

2. Guilleminault C, Dement WC. 235 cases of excessive daytime sleepiness. Diagnosis and tentative classification. J Neurol Sci. 1977. 31:13–27.
3. Joo EY, Hong SB, Kim HJ, Lim YH, Koo DL, Ji KH, et al. The effect of modafinil on cortical excitability in patients with narcolepsy: a randomized, placebo-controlled, crossover study. Sleep Med. 2010. 11:862–869.

4. Joo EY, Hong SB, Tae WS, Kim JH, Han SJ, Cho YW, et al. Cerebral perfusion abnormality in narcolepsy with cataplexy. Neuroimage. 2005. 28:410–416.

5. Joo EY, Tae WS, Kim JH, Kim BT, Hong SB. Glucose hypometabolism of hypothalamus and thalamus in narcolepsy. Ann Neurol. 2004. 56:437–440.

6. Joo EY, Tae WS, Kim ST, Hong SB. Gray matter concentration abnormality in brains of narcolepsy patients. Korean J Radiol. 2009. 10:552–558.

7. Naumann A, Daum I. Narcolepsy: pathophysiology and neuropsychological changes. Behav Neurol. 2003. 14:89–98.

8. Aguirre M, Broughton R, Stuss D. Does memory impairment exist in narcolepsy-cataplexy? J Clin Exp Neuropsychol. 1985. 7:14–24.

10. Rogers AE, Rosenberg RS. Tests of memory in narcoleptics. Sleep. 1990. 13:42–52.
11. Henry GK, Satz P, Heilbronner RL. Evidence of a perceptual-encoding deficit in narcolepsy? Sleep. 1993. 16:123–127.

12. Naumann A, Bellebaum C, Daum I. Cognitive deficits in narcolepsy. J Sleep Res. 2006. 15:329–338.

13. Andersson L, Allebeck P, Gustafsson JE, Gunnell D. Association of IQ scores and school achievement with suicide in a 40-year follow-up of a Swedish cohort. Acta Psychiatr Scand. 2008. 118:99–105.

14. Saltzman KM, Weems CF, Carrion VG. IQ and posttraumatic stress symptoms in children exposed to interpersonal violence. Child Psychiatry Hum Dev. 2006. 36:261–272.

15. Ryland HK, Lundervold AJ, Elgen I, Hysing M. Is there a protective effect of normal to high intellectual function on mental health in children with chronic illness? Child Adolesc Psychiatry Ment Health. 2010. 4:3.

16. Dorris L, Zuberi SM, Scott N, Moffat C, McArthur I. Psychosocial and intellectual functioning in childhood narcolepsy. Dev Neurorehabil. 2008. 11:187–194.

17. Ha KS, Yoo HK, Lyoo IK, Jeong DU. Computerized assessment of cognitive impairment in narcoleptic patients. Acta Neurol Scand. 2007. 116:312–316.

18. Jeon YS, Seo BY, Lee CW. KWIS Guide Book. 1963. Seoul: Institute for Better Education.
19. Kang YW, Kim JK. Korean-California Verbal Learning Test (K-CVLT). 2000. Seoul: Special Education.
20. Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial. 1995. Odessa, FL: Psychological Assessment Resources, Inc..
21. Wechsler D. Wechsler Memory Scale-Revised Manual. 1987. San Antonio, TX: The Psychological Corporation.
22. Corsi PM. Human memory and the medial temporal region of the brain. Diss Abstr Int. 1972. 34:819B.
23. Reitan RM. Trail Making Test: Manual for Administration and Scoring. 1992. Tucson, AZ: Reitan Neuropsychology Laboratory.
24. Lee YH, Song JY. A study of the reliability and the validity of the BDI, SDS, and MMPI-D scales. Korean J Clin Psychol. 1991. 10:98–113.
25. Cho YR, Kim JH. Confirmatory factor analysis of the Korean version of the Beck Depression Inventory: testing configural and metric invariance across undergraduate and clinical samples. Korean J Clin Psychol. 2002. 21:843–857.
26. Shek DT. Reliability and factorial structure of the Chinese version of the Beck Depression Inventory. J Clin Psychol. 1990. 46:35–43.

27. Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual Revised and Expanded. 1993. Odessa, FL: Psychological Assessment Resources, Inc..
28. Lee JH, Kang YW, Na DL. Efficiencies of stroop interference indexes in healthy older adults and dementia patients. Korean J Clin Psychol. 2000. 19:807–818.
29. Kang YW, Na DL. Seoul Neuropsychological Screening Battery. 2003. Seoul: Haman Brain Research & Consulting.
30. Raven JC. Coloured Progressive Matrices Sets A, Ab, B. Manual Sections 1 & 2. 1995. Oxford: Oxford Psychological Press.
31. Kim HH, Na DR. Korean Boston Naming Test (K-BNT). 1997. Seoul: Hak-Ji-Sa.
33. Neuropsychology Society. Neuropsychological Assessment. 2007. 3rd ed. Seoul: Korea Medical Book Publisher.
34. Szklo-Coxe M, Young T, Finn L, Mignot E. Depression: relationships to sleep paralysis and other sleep disturbances in a community sample. J Sleep Res. 2007. 16:297–312.

35. Schulz H, Wilde-Frenz J. Symposium: cognitive processes and sleep disturbances: the disturbance of cognitive processes in narcolepsy. J Sleep Res. 1995. 4:10–14.
36. Kim JE, Lee BR, Chun JE, Lee SJ, Lee BH, Yu IK, et al. Cognitive dysfunction in 16 patients with carotid stenosis: detailed neuropsychological findings. J Clin Neurol. 2007. 3:9–17.

37. Park YS. Practices of Psychological Assessment. 1994. Seoul: Ha-Na Medical.
38. Barnett JH, Salmond CH, Jones PB, Sahakian BJ. Cognitive reserve in neuropsychiatry. Psychol Med. 2006. 36:1053–1064.

39. Corral M, Rodríguez M, Amenedo E, Sánchez JL, Díaz F. Cognitive reserve, age, and neuropsychological performance in healthy participants. Dev Neuropsychol. 2006. 29:479–491.

41. Foley J, Garcia J, Shaw L, Golden C. IQ predicts neuropsychological performance in children. Int J Neurosci. 2009. 119:1830–1847.

42. Lim YR, Lee WK, Lee WH, Park JW. The study on the accuracy and validity of Korean Wechsler Intelligence Scale short forms: a comparison of the WARD7 subtest vs Doppelt subtest. Korean J Clin Psychol. 2000. 19:563–574.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download