Abstract
Migraine headache is commonly associated with signs of exaggerated intracranial and extracranial mechanical sensitivities. Patients exhibiting signs of intracranial hypersensitivity testify that their headache throbs and that mundane physical activities that increase intracranial pressure (such as bending over or coughing) intensify the pain. Patients exhibiting signs of extracranial hypersensitivity testify that during migraine their facial skin hurts in response to otherwise innocuous activities such as combing, shaving, letting water run over their face in the shower, or wearing glasses or earrings (termed here cephalic cutaneous allodynia). Such patients often testify that during migraine their bodily skin is hypersensitive and that wearing tight cloth, bracelets, rings, necklaces and socks or using a heavy blanket can be uncomfortable and/or painful (termed her extracephalic cutaneous allodynia). This review summarizes the evidence that support the view that activation of the trigeminovascular pathway contribute to the headache phase of a migraine attack, that the development of throbbing in the initial phase of migraine is mediated by sensitization of peripheral trigeminovascular neurons that innervate the meninges, that the development of cephalic allodynia is propelled by sensitization of second-order trigeminovascular neurons in the spinal trigeminal nucleus which receive converging sensory input from the meninges as well as from the scalp and facial skin, and that the development of extracephalic allodynia is mediated by sensitization of third-order trigeminovascular neurons in the posterior thalamic nuclei which receive converging sensory input from the meninges, facial and body skin.
Migraine is a heterogeneous neurological disorder characterized as a recurring, episodic, unilateral headache. Migraine pain usually throbs1 and typically intensifies during physical activities that increase intracranial pressure (e.g. bending over; coughing).2,3 The pain is associated with high incidence of nausea and predominance of hypersensitivity to light (photophobia) and noise (phonophobia). Symptoms of lesser prevalence include, aversion to odors (osmophobia), vomiting, fatigue, red eyes, tearing, nasal congestion, frequent yawning. A migraine attack may be precipitated by endogenous factors (i.e., hormonal changes, psychosocial stress, sleep deficit or surplus, hunger), or by exogenous factors (i.e., certain kinds of food; stimulation of different sensory modalities). An attack can be preceded by abnormal visual, sensory, motor and/or speech functions (migraine with aura) or start with no warning signs (migraine without aura).
For many years, migraine headache has been thought to be related to dilation of extracranial arteries. The theory was, as cited extensively in medical textbooks, that abnormal vasodilatation during migraine causes mechanical activation of perivascular stretch receptors, resulting in throbbing headache.4 This view was based on observations that extracranial arteries are vasodilated, edematous, and partially damaged during migraine.4,5 However, the extracranial vascular theory has fallen out of favor because clinical studied have yielded no convincing evidence for any significant extracranial vasodilation during migraine, nor have they shown that vasodilation can produce headache.6,7
The prevailing view today is that migraine headache is a neurovascular disorder of intracranial origin that involves meningeal blood vessels and the pain fibers that innervate them. This theory has originated from reports that electrical and mechanical stimulation of dural vasculature (but not the surface of the brain) produced referred head pain in awake patients undergoing craniotomy8,9: 1) periorbital pain - by stimulating the superior sagittal sinus or blood vessels of the floor of the anterior fossa; 2) parietal/temporal pain - by stimulating the middle meningeal artery; 3) occipital pain - by stimulating the dura at the floor of the posterior (PO) fossa, and the sigmoid, transverse and occipital sinuses. It should be emphasized that a) no sensation other than pain was evoked by stimulation of these structures; b) stimulation of non-vascular areas of the dura was largely ineffective in inducing pain sensation. These findings fit well with the pattern of dural innervation, whereby sensory nerves that originate in trigeminal and upper cervical ganglia closely follow meningeal blood vessels but not non-vascular areas of the dura.9 It was not until the 1980's that the nature of dural innervation was proved to be nociceptive. We now know that the dura is richly innervated by unmyelinated (C-fibers) and thinly myelinated (Aδ fibers) axons that originate in the trigeminal ganglion and in C1-3 dorsal root ganglions6,10-12 and that these pain fibers contain vasoactive neuropeptides such as substance P and calcitonin gene-related peptide (CGRP).13,14 These lines of evidence promoted the theory that the headache phase of migraine is mediated by activation of nociceptors that innervate meningeal blood vessels (i.e., meningeal nociceptors), and provided the basis for developing animal models of neurovascular head pain with intracranial origin.
The first animal model of neurovascular head pain employed the paradigm of electrical and/or mechanical stimulation of the dural sinuses.15,16 Using anatomical, physiological, histological and pharmaceutical techniques, such animal studies have demonstrated the presence of dura-sensitive neurons in brain and spinal cord structures, such as the medullary dorsal horn, thalamus, hypothalamus, periaqueductal gray (PAG).15,17-24 In the medullary dorsal horn25,26 and thalamus27,28 the majority of these trigeminovascular neurons respond to noxious skin stimuli either preferentially (wide-dynamic range neurons) or exclusively (high-threshold neurons). Administration of anti-migraine drugs such as ergotamines, triptans, or CGRP antagonists have been shown to inhibit responses to electrical stimulation of the dura in dura-sensitive neurons in the medullary dorsal horn.19,29-31
The acute stimulation of dural sinuses as a model for neurovascular head pain with intracranial origin has identified the trigeminovascular system and established the potential involvement of each of its elements in vascular headache and the associated symptoms. However, acute electrical or mechanical stimulation of the dura does not induce migraine in man, and can only evoke a short burst of activity in peripheral and central trigeminovascular neurons.
In recent years, it has become apparent that many types of prolonged or chronic pain are associated with long-lasting activation and sensitization of peripheral nociceptors and/or central nociceptive neurons in the dorsal horn. Incorporating these concepts into basic research on migraine pathophysiology, a new animal model for long-lasting headache of migraine was developed.
This model involves prolonged activation and subsequent sensitization of the trigeminovascular system in response to a brief exposure of the dura to a mixture of inflammatory agents consisting of serotonin, bradykinin, histamine, and prostaglandin.32 These agents activate and sensitize somatic and visceral nociceptors in the rat33-37 and are potent algesics in humans,38-41 capable of inducing headache.41
Using this animal model, it was found that a brief chemical irritation of the dura activates and sensitizes meningeal nociceptors (first-order trigeminovascular neurons) over a long period of time, rendering them responsive to mechanical stimuli to which they showed only minimal or no response prior to their sensitization (Fig. 1).32 During migraine, such peripheral sensitization is likely to mediate the throbbing pain and its aggravation during routine physical activities such as coughing, sneezing, bending over, rapid head shake, holding one's breath, climbing up the stairs, or walking.
By the end of migraine, when meningeal nociceptors are presumably no longer sensitized, their sensitivity to fluctuations in intracranial pressure returns to normal, and the patient no longer feels throbbing.
Brief stimulation of the dura with inflammatory agents also activates and sensitizes second-order trigeminovascular neurons located in the medullary dorsal horn that receive convergent input from the dura and the skin.25 In this paradigm, the central trigeminovascular neurons develop hypersensitivity in the periorbital skin, manifested as increased responsiveness to mild stimuli (brush, heat, cold) to which they showed only minimal or no response prior to their sensitization (Fig. 2). The induction of central sensitization by intracranial stimulation of the dura, and the ensuing extracranial hypersensitivity was taken to suggest that a similar process occurs in patients during migraine (Fig. 3).
Extracranial hypersensitivity during migraine was first noted in 187342 and later documented in the 1950's.5,43 At that time, extracranial hypersensitivity was ascribed to "hematomas that develop hours after onset of headache as a result of damage to vascular walls of blood vessels such as the temporal artery",5 or "widespread distension of extracranial blood vessels or spasm of suboccipital scalp muscles".43 The current view, however, is that extracranial hypersensitivity is a manifestation of central neuronal sensitization rather than extracranial vascular pathophysiology. Recent quantitative stimulation applied to the surface of the skin showed that pain thresholds to mechanical, heat, and cold skin stimuli decrease significantly during migraine in the majority of patients (Fig. 3A).44 This skin hypersensitivity, termed cutaneous allodynia, is typically found in the periorbital area on the side of the migraine headache. Patients commonly notice cutaneous allodynia during migraine when they become irritated by innocuous activities such as combing, shaving, taking a shower, wearing eyeglasses or earrings, or resting their head on the pillow on the headache side. Ipsilateral cephalic allodynia is likely to be mediated by sensitization of trigeminovascular neurons in the medullary dorsal horn that process sensory inputs from the dura and periorbital skin.
In the course of studying cephalic allodynia during migraine, we unexpectedly found clear evidence for allodynia in remote skin areas outside the innervation territory of the trigeminal nerve (Fig. 3B).44
In the discussion of that study, we proposed that ipsilateral cephalic allodynia is mediated by sensitization of dura-sensitive neurons in the medullary dorsal horn because their cutaneous receptive field is confined to innervation territory of the ipsilateral trigeminal nerve21,25,26,45-47 and that extracephalic allodynia must be mediated by neurons that process sensory information they receive from all levels of the spinal and medullary dorsal horn. Our search of such neurons focused on the thalamus since an extensive axonal mapping of sensitized trigeminovascular neurons in the spinal trigeminal nucleus revealed distinguish projections to the PO, the ventral posteromedial and the sub-parafascicular nuclei.
In 2010 we reported that topical administration of inflammatory molecules to the dura sensitized thalamic trigeminovascular neurons that process sensory information from the cranial meninges and cephalic and extracephalic skin.48 Sensitized thalamic neurons developed ongoing firing and exhibited hyper-responsiveness (increased response magnitude) and hypersensitivity (lower response threshold) to mechanical and thermal stimulation of extracephalic skin areas (Fig. 4).
Relevant to migraine pathophysiology was the finding that in such neurons, innocuous extracephalic skin stimuli that did not induce neuronal firing before sensitization (e.g., brush) became as effective as noxious stimuli (e.g., pinch) in triggering large bouts of activity after sensitization was established.
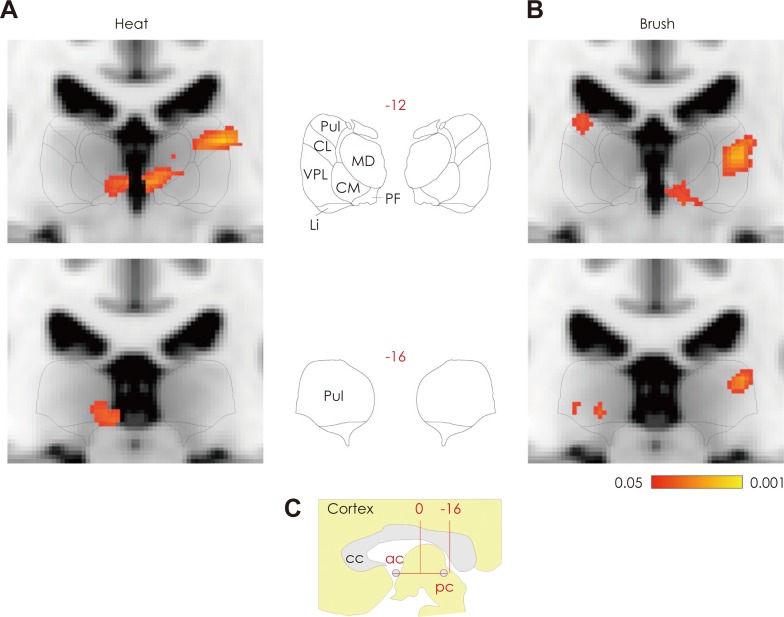
To understand better the transformation of migraine headache into widespread, cephalic and extracephalic allodynia, we also studied the effects of extracephalic brush and heat stimuli on thalamic activation registered by fMRI during migraine in patients with whole-body allodynia.48 Functional assessment of blood oxygenation level-dependent signals showed that brush and heat stimulation at the skin of the dorsum of the hand produced larger blood oxygenation level-dependent responses in the PO thalamus of subjects undergoing a migraine attack with extracephalic allodynia than the corresponding responses registered when the same patients were free of migraine and allodynia (Fig. 5).
Central sensitization can be either activity-dependent or activity-independent.49 The induction of sensitization in second-order trigeminovascular neurons, using chemical stimulation of the rat dura, is activity dependent, as evidenced by lidocaine blockade of afferent inputs from the dura. Once established, however, sensitization of the second-order trigeminovascular neurons becomes activity-independent, as it can no longer be interrupted by lidocaine on the dura.25 Translating theses findings in the context of migraine with allodynia, it appears that central sensitization depends on incoming impulses from the meninges in the early phase of the attack, and maintains itself in the absence of such sensory input later on. This view is strongly supported by the effects of the anti-migraine 5-HT1B/1D agonists, known as triptans, on the induction and maintenance of central sensitization in the rat,50 and the corresponding effects of early and late triptan therapy on allodynia during migraine.51 In the rat, triptan administration concomitant with chemical irritation of the dura effectively prevents the development of central sensitization. Similarly, treating patients with triptans early, within 60 min of the onset of migraine, effectively blocks the development of cutaneous allodynia. However, neither central neuronal sensitization in the rat, nor cutaneous allodynia in patients, can be reversed by late triptan treatment (2 hours after the application of sensitizing agent to the dura in the animal model, and 4 hours after the onset of migraine in allodynic patients). Most importantly, central sensitization appears to play a critical role in the management of migraine headache of allodynic patients. While non-allodynic patients can be rendered pain-free with triptans at any time during an attack, allodynic patients can be rendered pain-free only if treated with triptans early in the attack, namely, before the establishment of cutaneous allodynia.51
The findings that triptans cannot block ongoing sensitization in second-order trigeminovascular neurons is consistent with the evidence that these neurons do not posses the 5-HT1D receptor52 that mediates the neuronal action of these drugs. If triptans do not abort migraine in the presence of allodynia (i.e., central sensitization), how do they render the patient pain-free in the absence of allodynia? The simple option would be a peripheral action of triptans that suppresses of ongoing sensitization in the meningeal nociceptors that bombard the second-order neuron in the dorsal horn. However, the evidence shows that sensitized meningeal nociceptors are not inhibited by triptans.53 Thus, it appears that triptans abort migraine by a central, presynaptic action in the dorsal horn that blocks transmission of nociceptive signals between first- and second-order trigeminovascular neurons.
A growing body of evidence suggests that migraine patients are mostly non-allodynic during the first years of their migraine experience, and are eventually destined to develop allodynia during their migraine attacks.44,51,54 It is therefore possible that repeated migraine attacks over the years have cumulative adverse consequences on the function of the trigeminovascular pathway, one of which is susceptibility to develop central sensitization. The threshold for a central trigeminovascular neuron to enter a state of sensitization depends on the balance between incoming nociceptive signals and their modulation by spinal and supraspinal pathways. Many of the modulatory supraspinal pathways converge on the PAG and rostral ventromedial medulla (RVM).55 Recent imaging studies have shown that the PAG is activated during migraine56 and that it is deposited with abnormally high levels of iron in patients with a long history of migraine, suggesting an abnormal neuronal functioning.57 Abnormal PAG functioning can either enhance activity of RVM neurons that facilitate pain transmission in the dorsal horn, or suppress activity of RVM neurons that inhibit pain transmission in the dorsal horn.58 This may enhance excitability and, therefore, promote responses of second-order trigeminovascular neurons to incoming nociceptive signals from the meninges, resulting in a reduced threshold for entering a state of central sensitization. Furthermore, the transition from episodic to chronic migraine that occurs in some patients over the years may involve a shift in the underlying pathophysiology from transient to chronic state of sensitization. Altered functions of modulatory supraspinal pain pathways can contribute to this progression in migraine pathophysiology.
A rather radical view on migraine pathophysiology originated from a 1987 report suggesting that the PAG may constitutes a so-called "headache generator". In that study, 15 out of 175 pain patients (8.5%) developed migraine-like headache immediately after undergoing craniotomy for electrodes implantation at (or near) the PAG.59 This point of view has promoted an interpretation that activation of the PAG during migraine is the source, rather than the consequence, of migraine pain.56,60 However, numerous studies have yielded overwhelming evidence to disprove the concept of the PAG as a "headache generator". The first line of evidence is that persistent postoperative headache lasting 3 months or longer is routinely observed in 9-38% of patients undergoing craniotomy for a wide variety of procedures, with or without electrode placement.61-65 This crucial information was not available to Raskin et al.59 when they made the statement that "comparable headache syndromes have not been seen following craniotomy or burr hole placement performed for a variety of disorders."
The second line of evidence comes from neuroimaging studies in humans66-69 and Fos expression studies in animals,70 demonstrating that PAG activation occurs in numerous non-headache pain paradigms. This indicates that PAG activation is a universal consequence of nociceptor activation anywhere in the body, which is perfectly consistent with the multiple input arriving at the PAG from nociceptive neurons through the length of the spinal cord. The third line of evidence is that electrical stimulation of the PAG produces a general whole-body pain relief.71 This indicates that modulation of pain by the PAG is non-specific, which is consistent with the anatomical evidence that individual RVM neurons project to multiple segments of the spinal cord and terminate in the dorsal horn.72-75 Finally, the fourth line of evidence is that stimulation of the PAG or RVM cannot generate firing in spinothalamic tract neurons; it can only increase or decrease firing in responses to noxious stimulation of their peripheral receptive fields.58 Accordingly, it is activation of specific dorsal horn neurons by input they receive from peripheral nociceptors that determine where pain modulation is needed. In the case of migraine it is activation of trigeminovascular neurons in the medullary dorsal horn by inputs from meningeal nociceptors.
In spite of extensive research, the endogenous mechanisms by which meningeal nociceptors become activated have not yet been identified. We shall briefly review two hypotheses that attempt to describe endogenous cascades of events in the dura that may lead up to activation of meningeal nociceptors during the headache phase of migraine.
According to this hypothesis, meningeal nociceptors are activated by local release of endogenous inflammatory mediators through a process termed neurogenic inflammation.6,76 The process refers to a host of events, including local increase in blood flow, leakage of plasma protein from blood vessels, mast cell degranulation, and platelet aggregation. Neurogenic inflammation can be evoked experimentally by noxious sti-mulation of nociceptors that innervate a given tissue and the subsequent release of vasoactive neuropeptides such as substance P, neurokinin A, and CGRP.77 Similar markers of neurogenic inflammation and neuropeptide release have been induced in the rat dura by antidromic activation of meningeal afferents through electrical stimulation of the trigeminal ganglion.78,79 Support for the role of neurogenic inflammation in migraine comes from evidence that plasma protein extravasation and mast cell degranulation can be blocked in the rat by antimigraine drugs, such ergot alkaloids and triptans,78,80 and by non-steroidal anti-inflammatory agents, such as indomethacin and acetylsalicylic acid.81
The neurogenic inflammation hypothesis does not explain, however, what causes the initial activation of meningeal nociceptors that triggers neurogenic inflammation in the dura. The next hypothesis has been proposed in an attempt to address this dilemma.
Cortical spreading depression (CSD) refers to a wave of brief excitation followed by prolonged (15-30 min) inhibition of neuronal activity that propagates slowly across the cortex at a rate of 2-6 mm/min. This neural phenomenon was first observed by Leao in the cortex of anesthetized rabbits,82 and later correlated with localized changes in blood flow that spread through the cortex at a similar rate.83 CSD has been implicated in the pathophysiology of migraine on the basis of neuroimaging studies showing slowly-migrating changes in cortical blood flow in patients tested during the visual aura phase of migraine.83-86 Since aura precedes the onset of headache by 20-30 min, it was postulated that CSD may lead up to the initial activation of meningeal nociceptors. But how would abnormal cortical activity produce an impact on dural pain fibers and blood vessels across the pia mater and arachnoids?
Direct electrophysiological evidence for the activation of trigeminovascular neurons by CSD were reported recently,87,88 the CSD hypothesis relies on two suppositions that are based on two independent sets of observation. One assumption is that molecules such as potassium ions, hydrogen ions, and glutamate that are released extracellularly during CSD in the cortex86,89,90 diffuse through the overlying meninges and activate meningeal nociceptors. The other assumption is that the induction of neurogenic inflammation in the dura by CSD91 is mediated by antidromic axonal reflex that propagates through sensory trigeminal axons collateral that innervate both the pia and dura. This assumption may be consistent with the finding that sensory denervation of the meninges blocks CSD-induced neurogenic inflammation in the dura.91 Anatomical evidence for the existence of individual trigeminal axons that innervate both the pia and dura are now available.92
Based on current understanding of migraine-associated cutaneous allodynia and the most probable mechanism of action of triptans in migraine therapy, it is recommended that patients with allodynia take triptans as early as possible into the attack before the emergence of any sign of allodynia. This recommendation is consistent with patients' testimonies that triptans are much more likely to render them pain-free when taken early rather than late.
Nevertheless, most migraineures testify that they routinely delay treatment until attacks are fully developed or the pain is severe. Justifying the delayed treatment are concerns about side effects, addiction, limits on supply imposed by prescribers, cost, and most commonly waiting to see if headache develops into a severe migraine attack.93 For these patients, one way to terminate migraine with allodynia and fully developed central sensitization is parenteral administration of COX1/COX2 inhibitors.94 Infusion of the COX1/COX2 inhibitor ketorolac in allodynic patients who already missed the critical period for triptan therapy terminated both the headache and the allodynia provided that the patient had no history of using opioids to treat her/his migraines. In the rat, infusion of COX-1/COX-2 inhibitors blocked sensitization in meningeal nociceptors and suppressed ongoing sensitization in spinal trigeminovascular neurons, suggesting that parenteral nonsteroidal anti-inflammatory drug administration acts in the dorsal horn to inhibit the central neurons directly and reduce the synaptic input from the peripheral trigeminovascular neuron.94,95
Though impractical as a routine migraine therapy, parenteral nonsteroidal anti-inflammatory drug administration should be useful as a non-narcotic rescue therapy for migraine in the setting of the emergency department. Patients who use an opioid therapy over extended period of time are at high risk of developing medication-overuse headache and low response to non-narcotic drugs. The rational for recommending against the use of opioids in allodynic migraine patients is based on evidence that opioids can facilitate sensitization in the dorsal horn96-99 through: 1) upregulation of NMDA receptors function, 2) downregulation of glutamate transporters, 3) production of nitric oxide, 4) activation of spinal glia, and 5) increase extracellular level of prostaglandins.
References
1. Anthony M. The treatment of migraine--old methods, new ideas. Aust Fam Physician. 1993; 22:1434–1435. 1438–1439. 1442–1443. PMID: 8379882.
2. Blau JN, Dexter SL. The site of pain origin during migraine attacks. Cephalalgia. 1981; 1:143–147. PMID: 7346182.

3. Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population--a prevalence study. J Clin Epidemiol. 1991; 44:1147–1157. PMID: 1941010.

4. Wolff HG. Headache and Other Head Pain. 1963. 2nd ed. New York: Oxford University Press.
5. Wolff HG, Tunis MM, Goodell H. Studies on headache; evidence of damage and changes in pain sensitivity in subjects with vascular headaches of the migraine type. AMA Arch Intern Med. 1953; 92:478–484. PMID: 13091465.
6. Moskowitz MA, Macfarlane R. Neurovascular and molecular mechanisms in migraine headaches. Cerebrovasc Brain Metab Rev. 1993; 5:159–177. PMID: 8217498.
7. Zwetsloot CP, Caekebeke JF, Jansen JC, Odink J, Ferrari MD. Blood flow velocity changes in migraine attacks--a transcranial Doppler study. Cephalalgia. 1991; 11:103–107. PMID: 1860130.

8. Ray BS, Wolff HG. Experimental studies on headache. Pain-sensitive structures of the head and their significance in headache. Arch Surg. 1940; 41:813–856.
9. Penfield W, McNaughton F. Dural headache and innervation of the dura mater. Arch Neurol Psychiatry. 1940; 44:43–75.

10. Keller JT, Saunders MC, Beduk A, Jollis JG. Innervation of the posterior fossa dura of the cat. Brain Res Bull. 1985; 14:97–102. PMID: 3872702.

11. Andres KH, von Düring M, Muszynski K, Schmidt RF. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol (Berl). 1987; 175:289–301. PMID: 3826655.

12. Mayberg M, Langer RS, Zervas NT, Moskowitz MA. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981; 213:228–230. PMID: 6166046.

13. Uddman R, Edvinsson L, Ekman R, Kingman T, McCulloch J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: trigeminal origin and co-existence with substance P. Neurosci Lett. 1985; 62:131–136. PMID: 2415882.

14. Keller JT, Marfurt CF. Peptidergic and serotoninergic innervation of the rat dura mater. J Comp Neurol. 1991; 309:515–534. PMID: 1717522.

15. Strassman A, Mason P, Moskowitz M, Maciewicz R. Response of brainstem trigeminal neurons to electrical stimulation of the dura. Brain Res. 1986; 379:242–250. PMID: 3742223.

16. Davis KD, Dostrovsky JO. Activation of trigeminal brain-stem nociceptive neurons by dural artery stimulation. Pain. 1986; 25:395–401. PMID: 3748590.

17. Goadsby PJ, Zagami AS. Stimulation of the superior sagittal sinus increases metabolic activity and blood flow in certain regions of the brainstem and upper cervical spinal cord of the cat. Brain. 1991; 114:1001–1011. PMID: 2043937.

18. Kaube H, Keay KA, Hoskin KL, Bandler R, Goadsby PJ. Expression of c-Fos-like immunoreactivity in the caudal medulla and upper cervical spinal cord following stimulation of the superior sagittal sinus in the cat. Brain Res. 1993; 629:95–102. PMID: 8287282.

19. Zagami AS, Goadsby PJ, Edvinsson L. Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides. 1990; 16:69–75. PMID: 2250767.

20. Strassman AM, Mineta Y, Vos BP. Distribution of fos-like immunoreactivity in the medullary and upper cervical dorsal horn produced by stimulation of dural blood vessels in the rat. J Neurosci. 1994; 14:3725–3735. PMID: 8207485.

21. Davis KD, Dostrovsky JO. Responses of feline trigeminal spinal tract nucleus neurons to stimulation of the middle meningeal artery and sagittal sinus. J Neurophysiol. 1988; 59:648–666. PMID: 3351579.

22. Benjamin L, Levy MJ, Lasalandra MP, Knight YE, Akerman S, Classey JD, et al. Hypothalamic activation after stimulation of the superior sagittal sinus in the cat: a Fos study. Neurobiol Dis. 2004; 16:500–505. PMID: 15262261.

23. Malick A, Jakubowski M, Elmquist JK, Saper CB, Burstein R. A neurohistochemical blueprint for pain-induced loss of appetite. Proc Natl Acad Sci U S A. 2001; 98:9930–9935. PMID: 11504950.

24. Levy D, Strassman AM. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J Neurophysiol. 2002; 88:3021–3031. PMID: 12466427.

25. Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998; 79:964–982. PMID: 9463456.

26. Yamamura H, Malick A, Chamberlin NL, Burstein R. Cardiovascular and neuronal responses to head stimulation reflect central sensitization and cutaneous allodynia in a rat model of migraine. J Neurophysiol. 1999; 81:479–493. PMID: 10036252.
27. Zagami AS, Lambert GA. Stimulation of cranial vessels excites nociceptive neurones in several thalamic nuclei of the cat. Exp Brain Res. 1990; 81:552–566. PMID: 2226688.

28. Davis KD, Dostrovsky JO. Properties of feline thalamic neurons activated by stimulation of the middle meningeal artery and sagittal sinus. Brain Res. 1988; 454:89–100. PMID: 3409027.

29. Goadsby PJ, Hoskin KL. Inhibition of trigeminal neurons by intravenous administration of the serotonin (5HT)1B/D receptor agonist zolmitriptan (311C90): are brain stem sites therapeutic target in migraine? Pain. 1996; 67:355–359. PMID: 8951929.
30. Hoskin KL, Kaube H, Goadsby PJ. Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine. A c-Fos and electrophysiological study. Brain. 1996; 119:249–256. PMID: 8624686.
31. Lambert GA, Lowy AJ, Boers PM, Angus-Leppan H, Zagami AS. The spinal cord processing of input from the superior sagittal sinus: pathway and modulation by ergot alkaloids. Brain Res. 1992; 597:321–330. PMID: 1473003.

32. Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996; 384:560–564. PMID: 8955268.

33. Beck PW, Handwerker HO. Bradykinin and serotonin effects on various types of cutaneous nerve fibers. Pflugers Arch. 1974; 347:209–222. PMID: 4857072.
34. Davis KD, Meyer RA, Campbell JN. Chemosensitivity and sensitization of nociceptive afferents that innervate the hairy skin of monkey. J Neurophysiol. 1993; 69:1071–1081. PMID: 8492149.

35. Mizumura K, Sato J, Kumazawa T. Effects of prostaglandins and other putative chemical intermediaries on the activity of canine testicular polymodal receptors studied in vitro. Pflugers Arch. 1987; 408:565–572. PMID: 2439985.

36. Neugebauer V, Schaible HG, Schmidt RF. Sensitization of articular afferents to mechanical stimuli by bradykinin. Pflugers Arch. 1989; 415:330–335. PMID: 2622760.

37. Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992; 12:86–95. PMID: 1309578.

38. Armstrong D, Jepson JB, Keele CA, Stewart JW. Pain-producing substance in human inflammatory exudates and plasma. J Physiol. 1957; 135:350–370. PMID: 13406745.

39. Guzman F, Braun C, Lim RK. Visceral pain and the pseudaffective response to intra-arterial injection of bradykinin and other algesic agents. Arch Int Pharmacodyn Ther. 1962; 136:353–384. PMID: 13903244.
40. Hollander W, Michelson AL, Wilkins RW. Serotonin and antiserotonins. I. Their circulatory, respiratory, and renal effects in man. Circulation. 1957; 16:246–255. PMID: 13447168.
41. Sicuteri F. Vasoneuractive substances and their implication in vascular pain. Res Clin Stud Headache. 1967; 1:6–45.
42. Liveing E. On Megrim, Sick-Headache, and Some Allied Disorders: A Contribution to the Pathology of Nerve-Storms. 1873. London: J. and A. Churchill.
43. Selby G, Lance JW. Observations on 500 cases of migraine and allied vascular headache. J Neurol Neurosurg Psychiatry. 1960; 23:23–32. PMID: 14444681.

44. Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000; 47:614–624. PMID: 10805332.

45. Craig AD, Dostrovsky JO. Thermoreceptive lamina I trigeminothalamic neurons project to the nucleus submedius in the cat. Exp Brain Res. 1991; 85:470–474. PMID: 1716595.

46. Strassman AM, Potrebic S, Maciewicz RJ. Anatomical properties of brainstem trigeminal neurons that respond to electrical stimulation of dural blood vessels. J Comp Neurol. 1994; 346:349–365. PMID: 7995855.

47. Ebersberger A, Ringkamp M, Reeh PW, Handwerker HO. Recordings from brain stem neurons responding to chemical stimulation of the subarachnoid space. J Neurophysiol. 1997; 77:3122–3133. PMID: 9212262.

48. Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010; 68:81–91. PMID: 20582997.

49. Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003; 26:696–705. PMID: 14624855.

50. Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004; 55:27–36. PMID: 14705109.

51. Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004; 55:19–26. PMID: 14705108.

52. Potrebic S, Ahn AH, Skinner K, Fields HL, Basbaum AI. Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: implications for the selective antimigraine action of triptans. J Neurosci. 2003; 23:10988–10997. PMID: 14645495.

53. Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A. 2004; 101:4274–4279. PMID: 15016917.
54. Mathew NT. Early intervention with almotriptan improves sustained pain-free response in acute migraine. Headache. 2003; 43:1075–1079. PMID: 14629242.

56. Weiller C, May A, Limmroth V, Jüptner M, Kaube H, Schayck RV, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995; 1:658–660. PMID: 7585147.

57. Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001; 41:629–637. PMID: 11554950.

58. Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002; 25:319–325. PMID: 12086751.

59. Raskin NH, Hosobuchi Y, Lamb S. Headache may arise from perturbation of brain. Headache. 1987; 27:416–420. PMID: 3667258.

60. Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ. Brainstem activation specific to migraine headache. Lancet. 2001; 357:1016–1017. PMID: 11293599.

61. Schaller B, Baumann A. Headache after removal of vestibular schwannoma via the retrosigmoid approach: a long-term follow-up-study. Otolaryngol Head Neck Surg. 2003; 128:387–395. PMID: 12646842.

62. Harner SG, Beatty CW, Ebersold MJ. Headache after acoustic neuroma excision. Am J Otol. 1993; 14:552–555. PMID: 8296857.
63. Gökalp HZ, Arasil E, Erdogan A, Egemen N, Deda H, Cerçi A. Tentorial meningiomas. Neurosurgery. 1995; 36:46–51. discussion 51. PMID: 7708167.

64. Kaur A, Selwa L, Fromes G, Ross DA. Persistent headache after supratentorial craniotomy. Neurosurgery. 2000; 47:633–636. PMID: 10981750.

65. Gee JR, Ishaq Y, Vijayan N. Postcraniotomy headache. Headache. 2003; 43:276–278. PMID: 12603648.

66. Derbyshire SW, Jones AK, Creed F, Starz T, Meltzer CC, Townsend DW, et al. Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. Neuroimage. 2002; 16:158–168. PMID: 11969326.

67. Petrovic P, Ingvar M, Stone-Elander S, Petersson KM, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain. 1999; 83:459–470. PMID: 10568854.

68. Hsieh JC, Ståhle-Bäckdahl M, Hägermark O, Stone-Elander S, Rosenquist G, Ingvar M. Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: a positron emission tomography study. Pain. 1996; 64:303–314. PMID: 8740608.

69. Iadarola MJ, Berman KF, Zeffiro TA, Byas-Smith MG, Gracely RH, Max MB, et al. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain. 1998; 121:931–947. PMID: 9619195.

70. Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000; 53:95–104. PMID: 11033213.

71. Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977; 197:183–186. PMID: 301658.

72. Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978; 4:451–462. PMID: 216303.

73. Holstege G, Kuypers HG. The anatomy of brain stem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog Brain Res. 1982; 57:145–175. PMID: 7156396.

74. Martin GF, Vertes RP, Waltzer R. Spinal projections of the gigantocellular reticular formation in the rat. Evidence for projections from different areas to laminae I and II and lamina IX. Exp Brain Res. 1985; 58:154–162. PMID: 3987846.

75. Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995; 74:1742–1759. PMID: 8989409.

76. Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol. 1984; 16:157–168. PMID: 6206779.

77. Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988; 24:739–768. PMID: 3288903.

78. Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci. 1987; 7:4129–4136. PMID: 3694267.

79. Dimitriadou V, Buzzi MG, Moskowitz MA, Theoharides TC. Trigeminal sensory fiber stimulation induces morphological changes reflecting secretion in rat dura mater mast cells. Neuroscience. 1991; 44:97–112. PMID: 1771000.

80. Buzzi MG, Moskowitz MA. The antimigraine drug, sumatriptan (GR 43175), selectively blocks neurogenic plasma extravasation from blood vessels in dura mater. Br J Pharmacol. 1990; 99:202–206. PMID: 2158835.
81. Buzzi MG, Sakas DE, Moskowitz MA. Indomethacin and acetylsalicylic acid block neurogenic plasma protein extravasation in rat dura mater. Eur J Pharmacol. 1989; 165:251–258. PMID: 2776831.
82. Leão AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944; 7:359–390.
83. Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain. 1994; 117:199–210. PMID: 7908596.

84. Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001; 98:4687–4692. PMID: 11287655.

85. Bowyer SM, Aurora KS, Moran JE, Tepley N, Welch KM. Magnetoencephalographic fields from patients with spontaneous and induced migraine aura. Ann Neurol. 2001; 50:582–587. PMID: 11706963.

86. James MF, Smith JM, Boniface SJ, Huang CL, Leslie RA. Cortical spreading depression and migraine: new insights from imaging? Trends Neurosci. 2001; 24:266–271. PMID: 11311378.

87. Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. 2011; 69:855–865. PMID: 21416489.

88. Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci. 2010; 30:8807–8814. PMID: 20592202.

89. Brinley FJ Jr, Kandel ER, Marshall WH. Potassium outflux from rabbit cortex during spreading depression. J Neurophysiol. 1960; 23:246–256. PMID: 13804482.

90. Rapoport SI, Marshall WH. Measurement of Cortical Ph in Spreading Cortical Depression. Am J Physiol. 1964; 206:1177–1180. PMID: 14208962.

91. Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002; 8:136–142. PMID: 11821897.

92. Kosaras B, Jakubowski M, Kainz V, Burstein R. Sensory innervation of the calvarial bones of the mouse. J Comp Neurol. 2009; 515:331–348. PMID: 19425099.

93. Foley KA, Cady R, Martin V, Adelman J, Diamond M, Bell CF, et al. Treating early versus treating mild: timing of migraine prescription medications among patients with diagnosed migraine. Headache. 2005; 45:538–545. PMID: 15953272.

94. Jakubowski M, Levy D, Goor-Aryeh I, Collins B, Bajwa Z, Burstein R. Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache. 2005; 45:850–861. PMID: 15985101.

95. Jakubowski M, Levy D, Kainz V, Zhang XC, Kosaras B, Burstein R. Sensitization of central trigeminovascular neurons: blockade by intravenous naproxen infusion. Neuroscience. 2007; 148:573–583. PMID: 17651900.

96. Raith K, Hochhaus G. Drugs used in the treatment of opioid tolerance and physical dependence: a review. Int J Clin Pharmacol Ther. 2004; 42:191–203. PMID: 15124977.

97. Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005; 28:661–669. PMID: 16246435.

98. Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A. 1999; 96:7731–7736. PMID: 10393889.

99. Ozawa T, Nakagawa T, Shige K, Minami M, Satoh M. Changes in the expression of glial glutamate transporters in the rat brain accompanied with morphine dependence and naloxone-precipitated withdrawal. Brain Res. 2001; 905:254–258. PMID: 11423104.

Fig. 1
Sensitization of peripheral trigeminovascular neuron (meningeal nociceptor) believed to mediate intracranial hypersensitivity. A: Experimental setup. B: Development of mechanical hypersensitivity over time. IS: inflammatory soup.
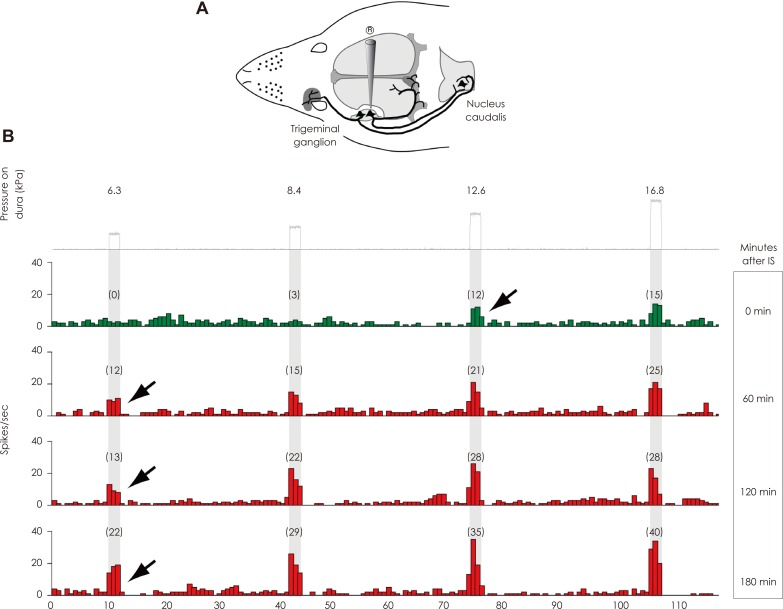
Fig. 2
Sensitization of central trigeminovascular neuron in the spinal trigeminal nucleus believed to mediate extracranial hypersensitivity and cephalic allodynia. A: Experimental setup. B: Recording site. C: Development of mechanical sensitization. D: Expansion of cephalic cutaneous receptive field. IS: inflammatory soup.
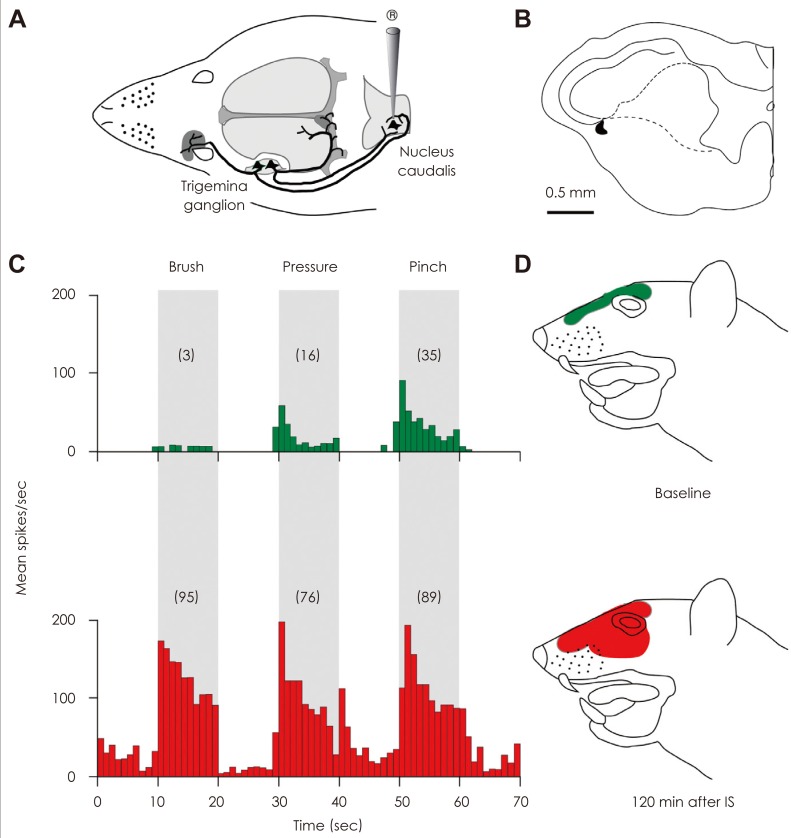
Fig. 3
Clinical presentation of cephalic and extracephalic allodynia during migraine. Number depict pain intensity, cold pain threshold, heat pain threshold and mechanical pain threshold. Areas shaded in green indicate values considered as allodynic. CL: contralateral, IL: ipsilateral, VFH: von-fray hair.
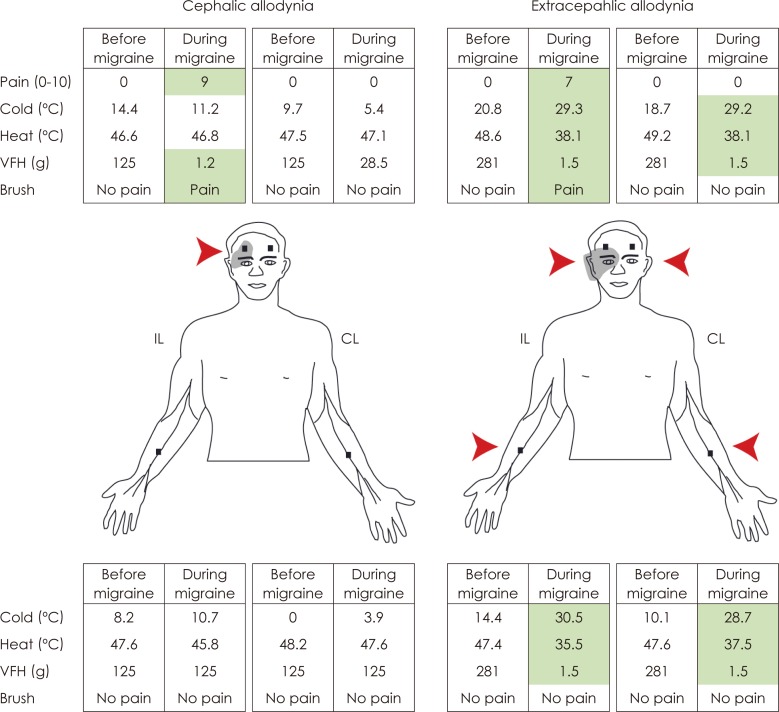
Fig. 4
Sensitization of central trigeminovascular neuron in the posterior thalamic nucleus believed to mediate extracranial allodynia. A: Expansion of cutaneous receptive field. B: Development of spontaneous activity. C: Enhanced responses to mechanical (top) and thermal (bottom) stimuli. Br: brush, H: habenula, IC: internal capsule, IS: inflammatory soup, Pi: pinch, PO: posterior thalamic nucleus, Pr: pressure, VPM: ventral posteromedial thalamic nucleus, VPL: ventral posterolateral thalamic nucleus.
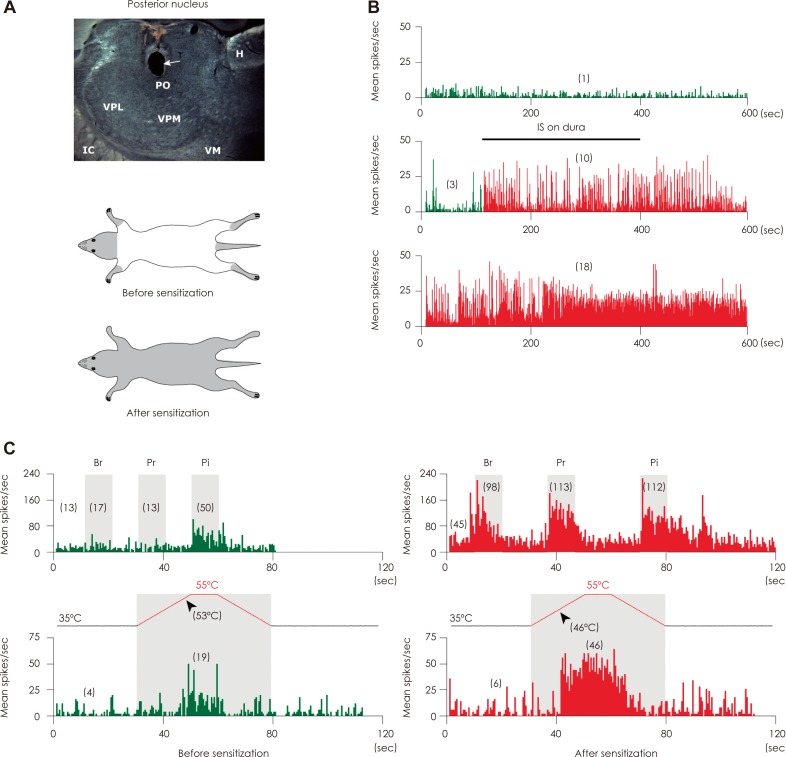
Fig. 5
Contrast analysis of blood oxygenation level-dependent signals registered in fMRI scans of the human posterior thalamus following innocuous and noxious skin stimuli during migraine attacks that were associated with extracephalic allodynia. ac: anterior commissure, cc: corpus callosum, CL: centrolateral thalamic nucleus, CM: centromedian thalamic nucleus, MD: mediodorsal thalamic group, Li: limitans thalamic nucleus, pc: posterior commissure, PF: parafascicular thalamic nucleus, Pul: pulvinar, VPL: ventral posterolateral thalamic.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download