Abstract
Background
Malignant peripheral nerve sheath tumors (MPNSTs), sarcomas originating from tissues of mesenchymal origin, are rare in patients without a history of neurofibromatosis.
Case Report
We report a case of an MPNST of the spinal accessory nerve, unassociated with neurofibromatosis, which metastasized to the brain. The tumor, originating in the intrasternomastoid segment of the spinal accessory nerve, was removed. Two years later, the patient presented with focal neurological deficits. Radiographic findings revealed a well-defined 2.2×2.2×2.2 cm, homogeneously enhancing mass in the left parieto-occipital region of the brain surrounded by significant vasogenic edema and mass effect, culminating in a 1-cm midline shift to the right. The mass was surgically removed. The patient had nearly complete recovery of vision, speech, and memory.
Malignant peripheral nerve sheath tumors (MPNSTs) are exceedingly rare in patients without a history of neurofibromatosis - a group of genetic disorders of the nervous system that cause tumors to grow on nerves and other tissues.1 MPNSTs of the spinal accessory nerve may involve the nerve at various points along its course from the cervical segment of the spinal cord to the trapezius muscle.2-7 The various segments of the nerve are classified as cisternal, foraminal, and intrasternomastoid.4,6 The extracranial portion of the nerve, specifically its intrasternomastoid segment, is the least common location of the nerve to be involved by a peripheral nerve sheath tumor,4-6 and metastasis to the brain from this location has not been reported. We report here a patient with a well-circumscribed mass in the brain parenchyma, which was a metastatic lesion of an MPNST of the intrasternomastoid segment spinal accessory nerve - a unique presentation of an exceeding rare tumor.
The patient is a 54-year-old white woman previously diagnosed as having an MPNST of the right spinal accessory nerve. Confirmation of the tumor was made by tissue biopsy of a mass located in the right supraclavicular region approximately 2 years ago. During her initial presentation in July 2009, we performed a wide excision of the mass and a sentinel lymph node biopsy of the right neck and axillary lymph nodes. Using a marking pen, we drew an elliptical incision around the mass to create a 2-cm margin from its palpable edge. In total, we excised an ellipse of tissue with diameters of 15×10 cm. After resection, the patient received four cycles of radiation therapy to the right supraclavicular region. She had been in good health since the diagnosis, and her outpatient medical regimen consisted only of a proton-pump inhibitor for treatment of gastroesophageal reflux disease. However, nearly 1 month before admission, the patient became symptomatic and presented to the emergency department with complaints of headache, visual disturbances, loss of short-term memory, and ataxic gait. In addition, she complained of visual loss on rightward gaze and loss of balance with head movement.
On presentation to the emergency department, her vital signs remained within normal limits. Physical examination revealed evidence of both long- and short-term memory deficits as well as a right visual field cut, and results of laboratory studies including an electrocardiogram were all within normal limits. However, radiographic examination of the brain by contrast-enhanced magnetic resonance imaging (MRI) revealed a well-defined 2.2×2.2×2.2 cm, homogeneously enhancing mass in the left parieto-occipital region surrounded by significant vasogenic edema. There was also evidence of mass effect, culminating in a 1-cm midline shift to the right; these findings were not present on an MRI obtained 5 months prior to admission (Fig. 1). The patient was subsequently admitted to the neurological intensive care unit (ICU) for neurological monitoring, and a neurosurgical consultation was obtained. As part of an evaluation for distant metastases, a contrast-enhanced CT scan of the chest revealed a new 1.1-cm nodule in the left lower lobe and a stable 0.4-cm nodule in the same location (Fig. 2). The patient's course in the neurological ICU was remarkable for only one episode of hypertension on the second day. Routine laboratory studies were obtained and were within normal limits.
On day 7 in the neurological ICU, we performed a craniotomy with complete removal of the mass. A biopsy of the mass was obtained in the operating room, and a pathologist confirmed the diagnosis of a metastatic nerve sheath tumor (Fig. 3). Histopathological findings revealed malignant tissue with poorly defined cellular and cystic components. The malignant cells expressed vimentin and S-100 but not glial fibrillary acidic protein, neurofilaments, melanoma marker, actin, smooth muscle antibody, pan-cytokeratin, or Melan-A. Confirmation of the brain tumor as a metastatic lesion of the original tumor of the spinal accessory nerve was based both on findings of immunohistochemical tests and the nearly identical morphological characteristics shared by the 2 tumors.
On postoperative day 1, the patient reported marked improvement in vision, speech, and memory, although not to baseline level. It is noteworthy that the brain lesion was considered to be a new tumor, because the results of an MRI scan of the brain obtained 5 months before this admission were within normal limits (Fig. 1B). The patient's postoperative course was uneventful and included a 2-week course of radiation therapy. On postoperative day 6, she was transferred to the oncology floor for further treatment.
To our knowledge, this is the first documented case of an MPNST arising from an extracranial segment of the spinal accessory nerve and metastasizing to the brain.1-4
Kurokowa et al.4 described the first case of a spinal accessory schwannoma arising in the fourth ventricle in a 50-year-old man presenting with neck pain after trauma. Physical examination findings from the patient described in their report were not consistent with cranial nerve involvement; and they did not report upper or lower extremity weakness or gait disturbance. The authors speculate that the accessory nerve tumor initially involved the intracisternal segment of the nerve and subsequently grew into the fourth ventricle. In their review of the literature, they identified only 30 cases of accessory schwannomas unassociated with neurofibromatosis published since 1975;4 however, only two of these cases presented with a schwannoma of the nerve in the region of the neck (i.e., intrasternomastoid portion), and neither of these cases presented with metastasis to the brain.6,7 Moreover, in both cases, no focal neurological deficits were reported on physical examination.6,7
MPNSTs are sarcomas originating from tissues of mesenchymal origin, i.e., peripheral nerves or cells of the nerve sheath such as Schwann cells or perineural cells.8 Hence, the terms malignant schwannoma, neurogenic sarcoma, or neurofibrosarcoma are used interchangeably.8-10 MPNST tumors commonly present a diagnostic challenge to pathologists because they arise from different types of cells (Schwann cells, fibroblasts, perineural cells) and may therefore produce a variable histological appearance.10 According to Geller and Gebhardt, at least one of the following has to be present for the diagnosis of MPNST: "1) a tumor arising from peripheral nerves; 2) a tumor arising from existing benign or other MPNST; or 3) a tumor that displays histological features of Schwann cells or perineural differentiation as revealed by immunohistochemical analysis or electron microscopic examination."10 MPNSTs comprise almost 10% to 12% of all soft tissue sarcomas, which are primarily associated with neurofibromatosis type 1 with a gene mutation of the same name; however, they can arise sporadically without neurofibromatosis type 1, in almost 50% of patients, as in the case of our patient. A strong relationship exists between a history of radiation therapy and MPNST.11,12 This tumor has a high percentage of recurrence at the primary or new site, and metastasis to the lungs, bones, or intra-abdominal or intrathoracic organs is not uncommon. However, metastasis to the brain is highly unusual; only a handful of cases have been reported to date, making this case of an MPNST of the spinal accessory nerve a novel finding.
MPNST usually occurs in patients between the ages of 20 to 50, but in an extreme case, it has been reported in an 11-month-old patient.9,10 These tumors present with pain, palpable mass, hemiparesis, or paresthesias, usually in a dermatomal pattern. Not surprisingly, an MPNST affects large peripheral nerves such as the sciatic nerve or the brachial or sacral plexus. Large nodular lesions in large peripheral nerves or plexus have greater potential for malignant transformation.10 Diagnosis and staging are usually done by surgical resection and biopsy. MRI and CT scans show the extent of the lesion and the distant metastasis and are therefore valuable in staging the lesion. Large tumors (>5 cm), invasion of fat planes, heterogeneity, ill-defined margins, and edema surrounding the lesion suggest an MPNST.13,14
According to Geller and Gebhardt, staging of soft tissue sarcomas is dependent on a number of factors including histological grade, tumor size, tumor depth, and whether or not metastases have been identified.10 In patients without detectable metastases, histological grade, tumor size, and tumor depth appear to predict subsequent metastasis.10 In addition, tumor staging is also based on the findings of imaging studies, which are frequently needed to define location and extent of disease.10,15
It is postulated that metastasis to the brain, though rare, happens by the following three routes:7 1) direct invasion; 2) hematogenous spread; 3) dissemination through the cerebrospinal fluid.16 It is not known whether the 9-mm pulmonary nodule identified on CT scans of the chest in the present case was a metastatic lesion, but by inference it raises the possibility of metastasis to the brain by a hematogenous route.16
It remains unclear why the brain is an infrequent site of metastasis. Although negative surgical margins and location of the tumor are considered important prognostic factors, patients frequently have metastases from negative surgical margins at the primary lesion site, as in the case of our patient. Radiation is still the mainstay of therapy because of high local recurrence and pulmonary involvement, and the role of chemotherapy in the treatment of this tumor still needs to be firmly established. The overall prognosis for a patient with this tumor is poor because most of the patients succumb to pulmonary complications; the 5-year survival rate ranges from 16% to 52%.16
Figures and Tables
Fig. 1
A: Initial MRI performed in 2009 as part of staging for peripheral nerve sheath tumor. T1 weighted axial image post gadolinium injection was negative for abnormal intracranial enhancement. B: Repeat MRI performed August 2010 on admission to neurological intensive care unit. T1 weighted axial image post gadolinium injection revealed 2.2×2.2×2.2 cm, well-circumscribed, homogeneously enhancing lesion identified in the left occipital lobe with extensive vasogenic edema. A midline shift to the right of approximately 1 cm with subfalcine herniation is evident. Mass effect is identified on the occipital horn of the lateral ventricle.
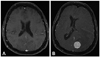
Fig. 2
Contrast-enhanced CT scan of the chest at the time of admission to the neurological intensive care unit. Scan revealed a new 1.1-cm left lower lobe solid nodular density adjacent to the lateral basilar segment artery and bronchus. A stable 0.4-cm nodule was seen peripheral to the left lower lobe.
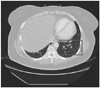
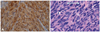
Fig. 3
A: Photomicrograph of an S-100 immunohistochemically stained slide (magnification ×400) of tissue from the original tumor, which confirms the tumor as an MPNST. B: Photomicrograph of an H & E slide (magnification ×400) of tissue from the cerebral mass. It shows a cellular neoplasm exhibiting storiform architecture with moderate nuclear atypia. Other areas show increased mitotic activity and necrosis. The diagnosis of MPNST was based on expression of similar immunohistochemical staining and nearly identical morphological characteristics shared between the original tumor and the metastatic tumor.
References
1. Fenzi F, Moretto G, Zamboni G, Passarin MG, Rizzuto N. Brain metastases from post-radiation malignant peripheral nerve sheath tumour. Ital J Neurol Sci. 1995. 16:495–498.

2. Tilgner J, Müller K, Ghanem N, Lutterbach J, Vesper J. Brain metastases as primary manifestation of a melanocytic malignant peripheral nerve sheath tumor in a 60-year-old man. BMC Neurol. 2007. 7:2.

4. Kurokawa R, Tabuse M, Yoshida K, Kawase T. Spinal accessory schwannoma mimicking a tumor of the fourth ventricle: case report. Neurosurgery. 2004. 54:510–514. discussion 514.

5. Agrawal A, Rao KS, Makannavar JH, Shetty L, Raveendra VM. Intrasternomastoid spinal accessory nerve schwannoma: clinical and radiological correlation. Neurol India. 2005. 53:347–348.

6. McShane D, Noyek AM, Chapnik JS, Steinhardt MI, Cooter N. Schwannoma of the intrasternomastoid portion of the spinal accessory nerve: sophisticated preoperative CT diagnosis and appropriate surgical management. J Otolaryngol. 1986. 15:282–285.
7. Noyek AM, Chapnik JS, Wortzman G, Kandel R. Schwannoma of the intrasternomastoid portion of the spinal accessory nerve: sophisticated pre-operative MRI diagnosis and appropriate surgical management. J Otolaryngol. 1992. 21:286–289.
8. D'Agostino AN, Soule EH, Miller RH. Sarcomas of the peripheral nerves and somatic soft tissues associated with multiple neurofibromatosis (von recklinghausen's disease). Cancer. 1963. 16:1015–1027.
9. Park SK, Yi HJ, Paik SS, Kim YJ, Ko Y, Oh SJ. Metastasizing malignant peripheral nerve sheath tumor initially presenting as intracerebral hemorrhage. Case report and review of the literature. Surg Neurol. 2007. 68:79–84. discussion 84.

10. Geller DS, Gebhardt M. Malignant peripheral nerve sheath tumors. Electronic Sarcoma Update Newsletter. 2006. Accessed 17 Feb 2011. Ossining, New York: Liddy Shriver Sarcoma Initiative;3. at http://sarcomahelp.org/learning_center/mpnst.html).
11. Adamson DC, Cummings TJ, Friedman AH. Malignant peripheral nerve sheath tumor of the spine after radiation therapy for Hodgkin's lymphoma. Clin Neuropathol. 2004. 23:245–255.
12. Amin A, Saifuddin A, Flanagan A, Patterson D, Lehovsky J. Radiotherapy-induced malignant peripheral nerve sheath tumor of the cauda equina. Spine (Phila Pa 1976). 2004. 29:E506–E509.

13. Friedrich RE, Kluwe L, Fünsterer C, Mautner VF. Malignant peripheral nerve sheath tumors (MPNST) in neurofibromatosis type 1 (NF1): diagnostic findings on magnetic resonance images and mutation analysis of the NF1 gene. Anticancer Res. 2005. 25:1699–1702.
14. Poyhonen M, Niemela S, Herva R. Risk of malignancy and death in neurofibromatosis. Arch Pathol Lab Med. 1997. 121:139–143.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download