Introduction
Methods
Participants
EEG recording
Stimuli and procedure
Average ERP analysis
Event-related spectral perturbation and inter-trial phase coherence
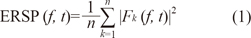
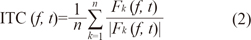
Journal List > J Clin Neurol > v.8(1) > 1048416
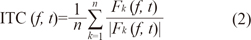
 | Fig. 1A: Grand averages showing N1 and N2 components evoked by a standard and a deviant stimulus in the oddball paradigm. The MMN is clearly elicited by subtracting the ERP evoked by a standard stimulus from the ERP evoked by a deviant stimulus. B: Voltage topographic mapping of each ERP component. ERP: event-related potential, MMN: mismatch negativity. |
 | Fig. 2A: Grand averages showing N1 and N2 components evoked by a standard and a deviant stimulus in the control paradigm. There is no discernible MMN component in the control paradigm. B: Voltage topographic mapping of each ERP component. ERP: event-related potential, MMN: mismatch negativity. |
 | Fig. 3ERSP in response to standard (A) and deviant (B) tones, and the difference between the two (C) in the oddball (upper row) and control (lower row) paradigms at the Fz electrode. The color of each image pixel indicates a significant change (p<0.001) of power (in dB) at a given frequency and latency relative to the baseline period (200 ms prior to stimulus onset). Note that the box in (C) indicates the TFOI for further statistical analysis. Topographic distributions of ERSPs from this box are depicted. ERSP: event-related spectral perturbations, TFOI: time-frequency of interest. |
 | Fig. 4ITC in response to standard (A) and deviant (B) tones, and the difference between the two (C) in the oddball (upper row) and control (lower row) paradigms at the Fz electrode. The color of each image pixel indicates a significant change (p<0.001) of power (in dB) at a given frequency and latency relative to the baseline period (200 ms prior to stimulus onset). Note that the box in (C) indicates the TFOI for further statistical analysis. Topographic distributions of the ITC from this box are depicted. ITC: inter-trial phase coherence, TFOI: time-frequency of interest. |