Abstract
Background
Lambert-Eaton myasthenic syndrome (LEMS) is a presynaptic neuromuscular junction disorder that is most frequently associated with small-cell lung cancer (SCLC). The titers of antibodies against voltage-gated calcium channels are frequently increased in LEMS, but only rarely is titer of anti-acetylcholine-receptor-binding antibodies (AChR-abs) increased.
Case Report
A 57-year-old male was admitted to our hospital due to dry mouth and eyes and progressive proximal limb weakness of 2 months duration. The results of a repetitive nerve stimulation test disclosed all criteria for the electrophysiological LEMS pattern, and the patient's AChR-abs titer was 0.587 nmol/L. At a follow-up performed 5 years after successful treatment of SCLC and LEMS, his AChR-abs titer had decreased to 0.001 nmol/L.
Lambert-Eaton myasthenic syndrome (LEMS) is a neuromuscular junction disorder characterized by fluctuating proximal limb muscle weakness, decreased deep-tendon reflexes, and various autonomic symptoms, and is most frequently associated with small-cell lung cancer (SCLC).1 The etiology of LEMS is the reduced exocytosis of acetylcholine from nerve endings by antibodies against voltage-gated calcium channels (VGCC-abs), increases in the titers of which are observed in more than 90% of patients with LEMS.1 It has been reported that titer of muscle anti-acetylcholine-receptor-binding antibodies (AChR-abs), which are more specific for myasthenia gravis (MG), is also increased in a small percentage of patients with LEMS (7%), although there are no detailed data or clinical information to corroborate this finding.1 Herein we present a case of LEMS with SCLC with increased AChR-abs titer (0.587 nmol/L), which had decreased to 0.001 nmol/L 5 years later during complete remission from LEMS.
A 57-year-old male was admitted to the hospital due to dry mouth and eyes and progressive proximal limb weakness without diurnal fluctuation of 2 months duration. He also complained of mild transient positional dizziness, dysarthria, and dysphagia. He had smoked one pack of cigarettes a day for 30 years. His family history was unremarkable. A neurologic examination showed mild dysarthria, proximal muscle weakness, and absent deep tendon reflexes. His cranial nerve, cerebellar, and sensory functions were all normal. A repetitive nerve stimulation test (RNST) was performed on the right abductor digiti quinti (ADQ), flexor carpi ulnaris (FCU), orbicularis oculi, nasalis, and trapezius muscles following Oh's method2 using the Toennies two-channel NeuroScreen system (Jaeger-Toennies, Hochberg, Germany). Postexercise facilitation (PEF) of compound muscle action potentials (CMAP) immediately after maximal voluntary contraction for 30 seconds and increment responses for 1 second at 50-Hz high-frequency stimulation were also recorded for the ADQ and FCU. The RNST results fulfilled all of the criteria for electrophysiological LEMS patterns: low-amplitude CMAP at rest, a decrement on low-frequency (3 Hz) stimulation, PEF of more than 100%, and an approximately 900% increment on high-frequency (50 Hz) stimulation (Fig. 1A).
Following an injection of acetylcholinesterase inhibitor (intramuscular injection of 1.5 mg of neostigmine methylsulfate into the deltoid muscle), arm elevation endurance improved from 30 seconds to 2 minutes, and this improvement lasted for up to 30 minutes postinjection; such an observation is an additional indicator of LEMS. The titer of AChR-abs was 0.587 nmol/L (normal: <0.2 nmol/L). Chest computed tomography (CT) revealed a 1.6-cm mass in the anterior segment of the right lower lobe and enlarged lymph nodes in the subcarina and lower paratracheal areas (Fig. 1B). SCLC was confirmed by a transbronchial lung biopsy. The patient was successfully treated with radiation (5.4 Gy for the primary tumor and regional lymph node area) and chemotherapy (irinotecan and cisplatin for the first cycle, followed by etoposide and cisplatin for the fifth cycle) for LEMS and SCLC, as confirmed by a follow-up electrophysiological examination (Fig. 1C) and chest CT (Fig. 1D) performed 13 months after the first evaluation. The patient was not treated for MG, but the titer of AChR-abs had decreased to 0.001 nmol/L at a follow-up performed 5 years after successful treatment.
It was clear that our patient had SCLC with LEMS as a paraneoplastic syndrome. Proximal limb muscle weakness, absence of deep tendon reflexes, dry mouth, and transient positional dizziness are clinical characteristics of LEMS. The diagnosis was supported by classic LEMS patterns on the RNST; the titers of VGCC-abs were not evaluated due to the cost of performing this test in Korea. The disappearance of the LEMS patterns on the RNST could be considered evidence of complete remission of SCLC.3
One unanswered question in this case is the clinical significance of the transiently increased titer of AChR-abs at the first evaluation. It is unclear whether or not this patient had MG. Clinical symptoms of dysarthria and dysphagia with definite improvement of muscle power after the injection of neostigmine are indicative of MG, but such characteristics are also often found in patients with LEMS.1 There have been several reports of electrophysiological LEMS patterns seen in otherwise typical seropositive MG.4-8 One of these studies found increased titers of VGCC-abs and transiently increased titer of AChR-abs, but the reported case did not have a malignant disease.8 To our knowledge, there has been only one reported case of lung cancer with LEMS and MG, but the clinical information is not sufficient to enable speculation as to the exact relationship between LEMS and AChR-abs.9 Moreover, although our patient was not treated with an immunosuppressant such as prednisolone or azathioprine, his symptoms and signs disappeared and he became seronegative for AChR-abs until after the cancer treatment.
Finally, we hypothesize that the transient 'nonpathologic epiphenomenon' of raised AChR-abs titer in SCLC with LEMS is more appropriate than true MG with spontaneous remission in this case. In addition to VGCC-abs, patients with LEMS with SCLC are seropositive for several autoantibodies to multiple organs; however, the clinical significance of this finding is unclear in most cases. Moreover, false-positive responses of AChR-abs have been reported in motor neuron disease with lung cancer, thymoma without MG, autoimmune hepatitis, graft-versus-host disease, and healthy relatives of patients with MG.10 The seronegative conversion of AChR-abs without treatment for MG supports our assumption. Further studies are needed to clarify the meaning of the seropositivity for AChR-abs in LEMS with SCLC.
Figures and Tables
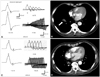 | Fig. 1Repetitive nerve stimulation test (RNST; A and C) and serial chest computed tomography (CT; B and D) and results before (A and B) and after (C and D) cancer treatment. The initial RNST (A) revealed electrophysiological patterns typical of Lambert-Eaton myasthenic syndrome: low compound muscle action potentials (1133.7 V; upper-left panel), postexercise facilitation (+121.1%; lower-left panel), a decrement (-65.7%) on low-frequency repetitive stimulation (3 Hz; upper-right panel), and an increment (+916%) on high-frequency repetitive stimulation (50 Hz; lower right panel) for the abductor digiti quinti. The initial chest CT (B) revealed a mass of approximately 1.6 cm in the anterior segment of the right lower lobe (white arrow). On follow-up studies at 13 months after treatment, the RNST responses had normalized (C) and the lung mass had completely disappeared on chest CT (D). |
References
1. Harper CM Jr, Lennon VA. Kaminski HJ, editor. Lambert-Eaton syndrome. Myasthenia Gravis and Related Disorders. 2009. 2nd ed. New York: Humana Press;209–225.

2. Oh SJ. Principles of Clinical Electromyography, Case Studies. 1998. Baltimore: Williams & Wilkins;21–22.
3. Lee SA, Sunwoo IN, Ro JK, Park KD. A case of remitted Lambert-Eaton myasthenic syndrome with small cell lung carcinoma following chemotherapy and radiotherapy. J Korean Neurol Assoc. 1992. 10:401–406.
4. Katz JS, Wolfe GI, Bryan WW, Tintner R, Barohn RJ. Acetylcholine receptor antibodies in the Lambert-Eaton myasthenic syndrome. Neurology. 1998. 50:470–475.

5. Newsom-Davis J, Leys K, Vincent A, Ferguson I, Modi G, Mills K. Immunological evidence for the co-existence of the Lambert-Eaton myasthenic syndrome and myasthenia gravis in two patients. J Neurol Neurosurg Psychiatry. 1991. 54:452–453.

6. Taphoorn MJ, Van Duijn H, Wolters EC. A neuromuscular transmission disorder: combined myasthenia gravis and Lambert Eaton syndrome in one patient. J Neurol Neurosurg Psychiatry. 1988. 51:880–882.

7. Tabbaa MA, Leshner RT, Campbell WW. Malignant thymoma with dysautonomia and disordered neuromuscular transmission. Arch Neurol. 1986. 43:955–957.

8. Oh SJ, Sher E. MG and LEMS overlap syndrome: case report with electrophysiological and immunological evidence. Clin Neurophysiol. 2005. 116:1167–1171.

9. Fettel M, Shin H, Penn A, Lovelace R, Rowland L. Combined Eaton-Lambert syndrome and myasthenia gravis. Neurology. 1978. 28:398.
10. Kaminski HJ, Santillan C, Wolfe GI. Autoantibody testing in neuromuscular disorders, part II: neuromuscular junction, hyperexcitability, and muscle disorders. J Clin Neuromuscul Dis. 2000. 2:96–105.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download