Abstract
Background and Purpose
White-matter (WM) lesions are known to potentiate cognitive impairment in poststroke patients. The present study was designed to assess whether Ginkgo biloba extract (GB) and cilostazol, which were evaluated alone and in a combination formula (Renexin), can attenuate the WM lesions and cognitive decline caused by chronic hypoperfusion in the rat.
Methods
Animals were divided into five treatment groups: cilostazol (25 mg/kg/day), GB (20 mg/kg/day), Renexin (25 mg/kg/day cilostazol + 20 mg/kg/day GB), vehicle, and sham. The animals received the treatments orally 1 day after bilateral common carotid artery occlusion [two-vessel occlusion (2VO); except for the sham group, which underwent the surgery but the arteries were not occluded], and then the same dose every day for 21 days thereafter. Prior to sacrificing the rats, repetitive eight-arm radial maze testing was performed to examine their cognitive abilities. After drug administration and cognitive testing, brain tissues were isolated for Klüver-Barrera and terminal deoxynucleotidyl transferase-mediated biotin-dUTP nick end-labeling (TUNEL) staining, immunohistochemical assessment of glial fibrillary acidic protein (GFAP) and CD11b (OX-42), and to assay free-radical scavenging activity.
Results
We found that the significant WM lesions induced by 2VO was ameliorated significantly by treatment with cilostazol, GB, and Renexin, in association with increased TUNEL-positive cells. In addition, chronic cerebral hypoperfusion caused a large increase in the degree of GFAP and OX-42 immunoreactivity and free-radical activity in the optic tract. These abnormalities were significantly reversed by the three drugs, but most prominently by Renexin, suggesting a markedly enhanced or supra-additive effect of cilostazol and GB when administered together.
Conclusions
Significant attenuation of cytoarchitectural damage and apoptotic cell death was found with GB and cilostazol, but a markedly enhanced effect was seen for treatment with their combination in the WM of rat brains after bilateral occlusion of the common carotid arteries. We suggest that combination therapy with GB and cilostazol provides enhanced neuroprotective effects and induces subsequent cognitive improvement in patients with chronic ischemic conditions.
A chronic, sustained reduction of cerebral blood flow can cause neuropathological changes that may lead to various neurological deficits such as chronic ischemic infarction or vascular dementia (VD) via the secondary impairment of cerebral glucose metabolism and chronic energy production.1 Unlike Alzheimer's disease, this pathology has implications because its incidence or severity can be lessened by thoroughly controlling stroke risk factors, such as hypertension, diabetes, or cardiac disorders.
According to previous clinical studies, VD patients exhibit more pronounced changes in the white matter (WM) than in the cortical gray matter (GM).2 Thus, WM lesions are known to play a more dominant role in the cognitive derangement observed in VD patients. Major pathological findings in WM under chronic cerebral hypoperfusion are microglial cell activation with myelin changes,3 whereas those in the cerebral cortex and hippocampus are delayed cell death with mild pathological changes and subsequent apoptosis.4,5
Ginkgo biloba extract (GB), which can increase blood flow by reducing blood viscosity and the elasticity of blood vessels, has been prescribed for controlling peripheral blood circulation disorders and ischemic encephalopathy. Flavonoids are a major component of GB, inhibiting enzymes such as cyclooxygenase A2, lipoxygenase, and phospholipase A2. GB also possesses anti-inflammatory properties, by scavenging the oxygen-free radicals that can be generated by stimulating the cytochrome P450 system.6 Terpene lactone, another component of GB, exerts antioxidant and anti-inflammatory effects and reduces blood coagulation. These elements behave as powerful antagonists of platelet-activating factor and exert a neuroprotective effect by reducing the increased free calcium in damaged cells, as well as reducing the production of oxygen free radicals in sensitized white blood cells and decreasing the production of nitric oxide.7,8
Cilostazol {6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydro-2-[1H]-quinolinone} is known to increase intracellular cyclic adenosine monophosphate levels by blocking its hydrolysis by type III phosphodiesterase (PDE3).9 Cilostazol can also scavenge hydroxyl and peroxyl radicals10 and suppress NAD(P)H oxidase-dependent superoxide production.11 Cilostazol was also recently acknowledged to possess neuroprotective anticytotoxic and antiapoptotic properties in focal cerebral ischemic models.12-14
Renexin, a mixed composition of GB and cilostazol, was recently introduced into clinical practice. In this study we investigated the neuroprotective effects of Renexin on chronic ischemic cerebral areas (especially WM) in a rat model with occlu-sion of the bilateral carotid arteries [two-vessel occlusion (2VO)], which may be different from those of cilostazol or GB alone.
All procedures used were approved by the Institutional Animal Care and Use Committee at the College of Medicine, The Catholic University of Korea. Male Wistar rats (10 weeks old), weighing 280±10 g (mean±SEM; Central Lab. Animal, Seoul, Korea) were used. The animals were housed in groups of 2-3 per cage at a temperature of 23±1℃ and a humidity of 50% with a 12/12-h light/dark cycle. Food and water were available ad libitum except when performing the eight-arm radial maze test.
All surgical procedures were carried out under inhalation anesthesia using 2% isoflurane with 30% O2 and 70% N2. Body temperature was monitored and maintained at 36-37℃ during the operation using a temperature controller. After fixing the rat on a board, a skin incision about 2 cm long was made and the bilateral common carotid arteries were exposed by isolating them from the vagus nerve and its sheath. They were then double ligated with 4-0 silk at 8-10 mm below the visible region of the external carotid artery. The sham group underwent the same surgical procedure but without ligation of their carotid arteries. All animals were allowed a recovery period of 10 days, during which they were provided with food and water ad libitum.
Cilostazol (99.0%) was obtained from Chemagis (Ramat Hovav Beer-Sheva, Israel). GB, an extract from G. biloba leaves, was provided by SK Chemicals (Seoul, Korea). All rats were randomly assigned to one of five treatment groups: 1) cilostazol (25 mg/kg/day), 2) GB (20 mg/kg/day), 3) Renexin (25 mg/kg/day cilostazol+20 mg/kg/day GB), 4) vehicle (0.5% carboxymethylcellulose), and 5) sham (0.5% carboxymethylcellulose). The animals received the drugs orally 1 day after the operation and then at the same dosage every day for 21 days after the surgery.
The spatial memory test was performed in an eight-arm radial maze comprising a center with a diameter of 34 cm and eight arms (60 cm long and 12 cm wide) with walls 20 cm high. All animals were trained twice a day for 10 days before the 2VO surgery so that they became familiar with the apparatus and the acquisition of food pellets. Among these animals, rats that experienced fewer than two total errors (TE) were selected for further testing. All of the animals were classified into five groups and received either permanent 2VO or the sham operation. At least 10 days prior to brain removal, measurements of the number of errors and the spent time were repeated in the same way (five times in total). The rats were then fed ad libitum for 3 h and then fasted. Four of the eight arms in the maze contained 40-50 mg of food pellets that were placed in the same place each time. Given the expected post-2VO visual loss, the floor paper in each arm had a different texture to allow the rats to identify the arms containing food. The rats were placed in the center of the maze, and a test was carried out for 10 min to see whether the rats ate all of the pellets.
A TE, reference memory error, or working memory error was considered to have occurred if the rat went to an arm without food, went to an arm without food for the first time, or returned to an arm already visited during the trial, respectively. The time taken to eat all of the food was recorded.
All rats were deeply anesthetized with 30% urethane that was perfused intracardially with 0.01 M phosphate-buffered saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer. The brain was extracted and fixed again with the same fixative for 4 h, and then soaked in a 30% sucrose solution until it sank. It was then snap-frozen with liquid nitrogen and stored at -70℃. Slices of 25 and 6 µm thick were then made by cutting at -0.26 to -0.4 mm and -2.8 to -3.14 mm posterior to the bregma using a cryostat microtome in accordance with the atlas of Paxinos.15
Each 25-µm-thick section was soaked in 0.1% Solvent Blue 38 (Sigma, USA) solution at 60℃ overnight, and the dye was removed (except for that in the WM region) using lithium carbonate solution, distilled water, and 70% ethanol. The section was soaked in 0.1% cresyl violet (Sigma, St. Louis, MO, USA) solution for 10 min, and then sequentially in 95% ethanol, 100% ethanol, and xylene for dehydration and clearing. After mounting, pathological findings in the WM (optic tract, internal capsule, anterior commissure, and corpus callosum) were observed using an optical microscope at a magnification of ×200. The severity of the WM lesion was graded as 0 (normal), 1 (disarranged nerve fibers), 2 (the formation of marked vacuoles), or 3 (disappearance of myelinated fibers), in accordance with a previous report.16
The terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling (TUNEL) assay was performed on 6-µm-thick brain sections including the WM using fluorescein with the In Situ Cell Death Detection Kit (Roche, Germany). TUNEL-positive cells were stained with 4',6-diamidino-2-phenylindole and then examined and counted with the aid of a fluorescence microscope.
Serial sections (6-µm thick) were cut on a cryostat microtome and the slides were incubated in 0.3% H2O2 for 10 min to quench endogenous peroxidase activity. Nonspecific antibody binding was then blocked with a blocking solution of 5% normal goat serum for 10 min. The preparations were incubated overnight at 4℃ with an antibody raised in rabbit against the astrocyte marker glial fibrillary acidic protein (GFAP; Dako, Glostrup, Denmark) and an antibody raised in mouse against the microglia marker CD11b (OX-42, Serotec, Oxford, UK), both diluted to 1:100 with antibody diluent (GBI Labs, Muki-lteo, WA, USA). They were then incubated with polymer hor-seradish peroxidase (Golden Bridge International, Inc., Los Angeles, CA, USA) for 10 min. The immunoreaction products were visualized using a solution of 0.02% 3,3'-diaminobenzidine tetrahydrochloride (polink-1 HRP kit, GBI Labs, Mukilteo, WA, USA), and then washed with distilled water and 0.01 M PBS. Finally, sections were counterstained with Mayer's hematoxylin (Scytek Laboratories, West Logan, UT, USA). Immunoreactivity for GFAP and OX-42 was quantified in the WM using the Image-Pro Plus Imaging software (Media Cybernetics, Silver Spring, MD, USA).
The malondialdehyde level (MDA)17 and the ratio of oxidized glutathione (GSSG) to total glutathione (GSHt), an indicator of lipid peroxidation, were measured at 14 days after the 2VO surgery with the Bioxytech LPO-586 assay kit (OxisResearch, Portland, OR, USA).
Rat brains were extracted and homogenized in 20 mM ice-cold Tris buffer (pH 7.4); 0.5 M butylated hydroxytoluene (Sigma-Aldrich) was then added to prevent oxidation. The homogenate was centrifuged at 3000×g for 10 min at 4℃. Next, 200 µL of the prepared sample was mixed with 650 µL of N-methyl-2-phenylindole (OxisResearch) and 150 µL of 37% HCl, both added continuously to the mixture. The reaction proceeded at 45℃ for 60 min, and the mixture was then centrifuged at 15000×g for 10 min. The concentration of MDA in the supernatant was measured by reading the absorbance at 586 nm with a spectrometer.
The GSSG/GSHt ratio was estimated using a glutathione (GSH) assay kit (Cayman, Ann Arbor, MI, USA). First, 100 µL of the diluted homogenate was mixed with 100 µL of 10% metaphosphoric acid (Sigma-Aldrich), and the mixed sample was then centrifuged at 2000×g for 2 min, and 100 µL of the supernatant was mixed with 1 µL of 1 M 2-vinylpyridine (Sigma-Aldrich). A GSH sample was prepared using the same method as for the preparation of the GSSG sample, but with 5 µL of 4 M triethanolamine reagent (Sigma-Aldrich) instead of 1 M 2-vinylpyridine. The absorbances of the GSSG and GSHt samples were measured at 405 nm using a spectrophotometer.
The results were analyzed using SigmaStat (ver. 3.5) and SPSS (ver. 12.0) software. Data are presented as mean±SEM values. Statistical analysis was performed by repeated one-way ANOVA or one-way ANOVA test (the Tukey test), Kruskal-Wallis one-way test (Dunn's test), t-test, and Mann-Whitney U statistics. Differences between groups were deemed to be statistically significant when p<0.05.
Among the 113 rats that underwent permanent 2VO, 39 (30%) died within 21 days after surgery. The rats that survived exhibited decreased body weight or transient difficulties in feeding. Some animals exhibited symptoms of transient ptosis.
Five groups of Wistar rats were examined for spatial memory on 5 consecutive days after day 21 following the 2VO surgery (cilostazol, n=10; sham, n=6; the other groups, n=9). Compared with the results of the vehicle group, there was a significant reduction in the TE score and the time spent in the maze arms in the GB and Renexin groups during the spatial memory test. In addition, the reference memory error scores decreased more in the Renexin group than in the cilostazol and GB groups (Fig. 1).
We evaluated the histological findings after ischemic insult in WM areas (optic tract, anterior commissure, corpus callosum, and internal capsule) in brain slices (25 µm thickness) from each group (Fig. 2A). The WM changes (formation of marked vacuoles and the disappearance of myelinated fibers) in the optic tract region were more severe in the vehicle group than in both the sham and the other groups (Fig. 2B), and appeared to be less prominent in the Renexin group than in the cilostazol group. The histological changes in the internal capsule and corpus callosum appeared to be greater in the GB group than in the cilostazol and Renexin groups, although the differences were not statistically significant (p>0.05) (Fig. 3).
To assess the degree of apoptosis, we counted TUNEL-positive cells on sections obtained between -2.8 mm and -3.3 mm from the bregma. The TUNEL-positive cell count was much higher in the vehicle group (31.3±17.38) than in the sham group (5.3±5.03; p<0.01; Fig. 4). However, a post-hoc analysis among the five groups disclosed no statistically significant differences between the groups (Renexin, 9.3±1.52 cells; GB, 10.33±6.50 cells; cilostazol, 17±3 cells).
Generally, the level of GFAP staining (marker for astrocytes) after chronic cerebral hypoperfusion was increased markedly in the vehicle group compared to the other groups (vs. sham group, p<0.001; vs. the other three groups, p<0.01). In particular, the GFAP level was significantly lower in the Renexin group (65.00±42.54) than in the cilostazol (89.37±47.08) and GB groups (78.28±39.84; post-hoc analysis, p<0.05) (Figs. 5A and 6). Immunohistochemical staining for OX-42, a marker for microglia, differed significantly between the Renexin group (82.83±65.94) and the cilostazol (122.5±67.27) and GB (107.66±55.53, p<0.01) groups (Figs. 5B and 6).
The levels of free radicals (GSSG/GSHt) were assessed on the 21st day after surgery to establish the success of the 2VO model. The free-radical levels were significantly lower in the cilostazol, GB, and Renexin groups than in the vehicle group (0.28±0.09, p<0.05) (Fig. 7A), but did not differ significantly among the three drug-administered groups. The MDA level was also lower in the cilostazol, GB, and Renexin groups than in the vehicle group, and also did not differ significantly among the three treatment groups (p>0.05) (Fig. 7B).
In the present investigation, cilostazol, GB, and Renexin exerted an inhibitory effect on cognitive decline in the radial maze test and a neuroprotective effect with respect to WM lesions (Klüver-Barrera stain) compared with the control treatments. The number of TUNEL-positive cells was higher in the vehicle group than in the sham group, but was lower in the drug-administered groups. The number of microglia and astrocytes increased markedly after establishment of the 2VO model, as assessed using immunohistochemistry with GFAP and OX-42. However, the numbers decreased after administration of the drugs. Finally, the drug-administered groups exhibited a decreasing tendency for free-radical scavenger activity relative to the control groups.
The experimental rat model induced by the permanent occlusion of the bilateral common carotid arteries (2VO) is an established model of VD.18-21 Tsuchiya et al.22 reported decreased cerebral blood flow in 15 areas (including the cortex, hippocampus, and caudate) among 24 candidate cerebral GM regions in 2VO-model rats over a 1-week period. These regions are known to have roles in learning and memory. In addition, marked ischemic changes were observed simultaneously in the WM, including the optic nerve, optic tract, corpus callosum, internal capsule, anterior commissure, and traversing fiber bundles of the caudoputamen.21
We observed that in rat brains subjected to chronic cerebral hypoperfusion by 2VO for 21 days there was a formation of marked vacuoles and a disappearance of myelinated fibers in the WM, including the optic tract, in association with an increased population of TUNEL-positive cells, all of which were significantly ameliorated by treatment with cilostazol, GB, and their combination (in Renexin). Furthermore, chronic cerebral hypoperfusion caused a large increase in the amount of GFAP and OX-42 immunoreactivity and free-radical activity in the optic tract. These abnormalities were also significantly reversed by the two drugs and their combination.
The 2VO model is known to induce damage in various WM (but not GM) structures, especially along cholinergic periventricular fibers and long association tracts.23,24 Our data are consistent with previous reports of histological findings and cognitive changes measured by radial maze tests.
Cilostazol may prevent cognitive impairment by suppressing the early accumulation of lipid peroxidation products, with the elimination of the sequential inflammatory responses, such as decreasing the number of apoptotic cells.25 Another pharmacological effect of cilostazol is that not only does it strongly scavenge hydroxyl radicals and reactive oxygen species (ROS) and decreases elevated tumor necrosis factor-α levels, but it also reduces the level of apoptosis and cell death, in parallel with an increase in Bcl-2 expression and a decrease in Bax protein levels and cytochrome c release.10
GB is known to be involved in mediating free-radical scavenging and antioxidant actions. It also exhibits neuroprotective effects, such as protection against Aβ toxicity, and antiapoptotic properties, probably due to protective effects on mitochondria.26
Interestingly, although cilostazol and GB lessened the morphological and neurobehavioral deteriorations induced by the 2VO model, respectively, Renexin appeared to be supra-additive neuroprotective in this study (as evidenced by WM staining with Klüver-Barrera staining and the radial maze test, respectively). In addition, there was no statistical significance between Renexin and cilostazol or GB with respect to TUNEL-positive cell counts, immunohistochemical staining (GFAP and OX-42), and free-radical levels (GSSG/GSHt and MDA). However, we found that the results were more favorable and consistent with Renexin than with cilostazol or GB.
We suggest that the combination of cilostazol and GB in Renexin exerts an enhanced therapeutic effect, with cilostazol (a PDE3 inhibitor) treating cerebrovascular WM lesions via hyperactivated astroglia or microglia immunoreactivity, and GB protecting and/or rescuing neuronal cells against various toxic conditions, such as elevated ROS.
A recent study produced the intriguing finding that a combination of cilostazol and GB was not accompanied by an increased bleeding tendency, but that the enhancement of the antiplatelet effects of cilostazol by GB was more pronounced in in vivo models.27 Although the exact mechanism underlying this supra-additive improvement remains unclear, this is the first report of a markedly enhanced neuroprotective effect from a combination of cilostazol and GB.
In conclusion, we have demonstrated that Renexin, comprising a combination of cilostazol and GB, not only potentiated the individual neuroprotective effects of the two drugs (manifesting as attenuated WM lesions) but also reduced cognitive decline caused by chronic hypoperfusion in the rat 2VO model used in this study. Furthermore, we suggest that Renexin has therapeutic utility in VD patients.
Figures and Tables
Fig. 1
The effects of cilostazol (25 mg/kg/day), GB (20 mg/kg/day), and renexin (25 mg/kg/day cilostazol+20 mg/kg/day GB) on rats that have undergone 2VO (of the common carotid arteries) performing the eight-arm radial maze test. Temporal changes are plotted as presurgery (days -3, -2, and -1), surgical operation (day 0), and postsurgery (days 17, 18, 19, 20, and 21) on the x-axis. Data are mean and SEM values for 9 or 10 animals. All data were compared between multiple groups. Statistical analysis was conducted using repeated one-way ANOVA followed by the Tukey test. A: Total error. *p<0.05 vs. vehicle; †p<0.05 vs. Renexin. B: Spent time (sec). *p<0.05 vs. vehicle; †p<0.05 vs. Renexin. C: Reference memory error. *p<0.05 vs. vehicle; †p<0.05 vs. Renexin. GB: Ginkgo biloba extract, 2VO: two-vessel occlusion.

Fig. 2
Klüver-Barrera staining for WM damage. A: Schematic representation of the rat brain at -0.30 mm and -3.30 mm from the bregma. B: Photographs of Klüver-Barrera staining in the optic tract (opt), anterior commissure (ac), corpus callosum (cc), and internal capsule (ic) of rats subjected to the sham operation, vehicle (0.5% carboxymethylcellulose), cilostazol (25 mg/kg/day), GB (20 mg/kg/day), and Renexin (25 mg/kg/day cilostazol+20 mg/kg/day GB). Scale bar=100 µm. GB: Ginkgo biloba extract.
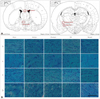
Fig. 3
Klüver-Barrera staining for white-matter damage. Data are mean and SEM values for 9 or 10 animals. All data were compared between multiple groups. Statistical analysis was conducted using one-way ANOVA followed by the Tukey test. ***p<0.001, **p<0.01, *p<0.05 vs. vehicle. The t-test method was used for statistical analysis with Renexin; †p<0.05 vs. renexin. ac: anterior commissure, cc: corpus callosum, GB: ginkgo biloba extract, ic: internal capsule, opt: optic tract.

Fig. 4
Representative photomicrographs of the terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling (TUNEL)-positive cells in the rat brain after ischemic insult (2VO). No TUNEL-positive cells were observed in sham-operated animals, but they were increasingly observed in the vehicle-treated hypoperfusion group. Data are mean and SEM values for seven animals. Statistical analysis was conducted using one-way ANOVA followed by the Tukey test. *p<0.05 vs. vehicle. The t-test method was used for statistical analysis with Renexin; †p<0.05 vs. renexin. Scale bar=50 µm.

Fig. 5
A: Immunohistochemical staining of glial fibrillary acidic protein as a marker for astrocytes. Data are mean and SEM values for seven animals. Statistical analysis was conducted using one-way ANOVA followed by the Tukey test; *p<0.01, **p<0.001 vs vehicle. B: Immunohistochemical staining of CD11β (OX-42) as a marker for microglia. Data are mean and SEM values for seven animals. Statistical analysis was conducted using one-way ANOVA followed by the Tukey test. *p<0.05 vs. vehicle. GB: Ginkgo biloba extract.

Fig. 6
Representative photomicrographs of GFAP and OX-42 immunostaining in the optic tract. Scale bar=50 µm. GB: Ginkgo biloba extract, GFAP: glial fibrillary acidic protein.

Fig. 7
Measurement of free-radical levels in the rat brain by assessing (A) GSSG/GSHt and (B) MDA levels. Data are mean and SEM values for seven animals. Statistical analysis was conducted using one-way ANOVA on rank (Kruskal-Wallis one-way ANOVA on ranks) followed by Dunn's test. *p<0.05 vs vehicle. GB: Ginkgo biloba extract, GSSG/GSHt: oxidized glutathione/total glutathione, MDA: malondialdehyde level.

References
1. Beal MF, Hyman BT, Koroshetz W. Do defects in mitochondrial energy metabolism underlie the pathology of neurodegenerative diseases? Trends Neurosci. 1993. 16:125–131.

2. Kim JS, Yun I, Choi YB, Lee KS, Kim YI. Ramipril protects from free radical induced white matter damage in chronic hypoperfusion in the rat. J Clin Neurosci. 2008. 15:174–178.

3. O'Brien MD. How does cerebrovascular disease cause dementia? Dementia. 1994. 5:133–136.
4. Bennett SA, Tenniswood M, Chen JH, Davidson CM, Keyes MT, Fortin T, et al. Chronic cerebral hypoperfusion elicits neuronal apoptosis and behavioral impairment. Neuroreport. 1998. 9:161–166.

5. Nanri M, Miyake H, Murakami Y, Matsumoto K, Watanabe H. Chronic cerebral hypoperfusion-induced neuropathological changes in rats. Nihon Shinkei Seishin Yakurigaku Zasshi. 1998. 18:181–188.
6. Szabo ME, Droy-Lefaix MT, Doly M. EGb 761 and the recovery of ion imbalance in ischemic reperfused diabetic rat retina. Ophthalmic Res. 1995. 27:102–109.

7. Pincemail J, Dupuis M, Nasr C, Hans P, Haag-Berrurier M, Anton R, et al. Superoxide anion scavenging effect and superoxide dismutase activity of Ginkgo biloba extract. Experientia. 1989. 45:708–712.

8. Varga E, Bodi A, Ferdinandy P, Droy-Lefaix MT, Blasig IE, Tosaki A. The protective effect of EGb 761 in isolated ischemic/reperfused rat hearts: a link between cardiac function and nitric oxide production. J Cardiovasc Pharmacol. 1999. 34:711–717.

9. Kimura Y, Tani T, Kanbe T, Watanabe K. Effect of cilostazol on platelet aggregation and experimental thrombosis. Arzneimittelforschung. 1985. 35:1144–1149.
10. Kim KY, Shin HK, Choi JM, Hong KW. Inhibition of lipopolysaccharide-induced apoptosis by cilostazol in human umbilical vein endothelial cells. J Pharmacol Exp Ther. 2002. 300:709–715.

11. Shin HK, Kim YK, Kim KY, Lee JH, Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation. 2004. 109:1022–1028.

12. Choi JM, Shin HK, Kim KY, Lee JH, Hong KW. Neuroprotective effect of cilostazol against focal cerebral ischemia via antiapoptotic action in rats. J Pharmacol Exp Ther. 2002. 300:787–793.

13. Lee JH, Lee YK, Ishikawa M, Koga K, Fukunaga M, Miyakoda G, et al. Cilostazol reduces brain lesion induced by focal cerebral ischemia in rats--an MRI study. Brain Res. 2003. 994:91–98.

14. Davidson CM, Pappas BA, Stevens WD, Fortin T, Bennett SA. Chronic cerebral hypoperfusion: loss of pupillary reflex, visual impairment and retinal neurodegeneration. Brain Res. 2000. 859:96–103.

15. Paxinos G. The Rat Brain in Stereotaxic Coordinates. 2007. 6th ed. San Diego: Academic Press.
16. Wakita H, Tomimoto H, Akiguchi I, Kimura J. Dose-dependent, protective effect of FK506 against white matter changes in the rat brain after chronic cerebral ischemia. Brain Res. 1998. 792:105–113.

17. Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991. 11:81–128.

18. Ihara M, Tomimoto H, Kinoshita M, Oh J, Noda M, Wakita H, et al. Chronic cerebral hypoperfusion induces MMP-2 but not MMP-9 expression in the microglia and vascular endothelium of white matter. J Cereb Blood Flow Metab. 2001. 21:828–834.

19. Murakami Y, Ikenoya M, Matsumoto K, Li H, Watanabe H. Ameliorative effect of tacrine on spatial memory deficit in chronic two-vessel occluded rats is reversible and mediated by muscarinic M1 receptor stimulation. Behav Brain Res. 2000. 109:83–90.

20. Tanaka K, Ogawa N, Asanuma M, Kondo Y, Nomura M. Relationship between cholinergic dysfunction and discrimination learning disabilities in Wistar rats following chronic cerebral hypoperfusion. Brain Res. 1996. 729:55–65.

21. Wakita H, Tomimoto H, Akiguchi I, Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol. 1994. 87:484–492.

22. Tsuchiya M, Sako K, Yura S, Yonemasu Y. Local cerebral glucose utilisation following acute and chronic bilateral carotid artery ligation in Wistar rats: relation to changes in local cerebral blood flow. Exp Brain Res. 1993. 95:1–7.

23. van den Heuvel DM, ten Dam VH, de Craen AJ, Admiraal-Behloul F, Olofsen H, Bollen EL, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry. 2006. 77:149–153.

24. Volbracht C, van Beek J, Zhu C, Blomgren K, Leist M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci. 2006. 23:2611–2622.

25. Watanabe T, Zhang N, Liu M, Tanaka R, Mizuno Y, Urabe T. Cilostazol protects against brain white matter damage and cognitive impairment in a rat model of chronic cerebral hypoperfusion. Stroke. 2006. 37:1539–1545.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download