Abstract
Background
Neurologic manifestations of primary Sjögren's syndrome (PSS) have been reported to vary from sensory polyneuropathy to encephalopathy or psychiatric problems. However, marked cerebellar degeneration associated with PSS has rarely been reported.
Case Report
We describe a patient with Sjögren's syndrome who exhibited rapidly progressive cerebellar ataxia, nystagmus, cognitive decline, and psychiatric problems. Brain magnetic resonance imaging revealed marked atrophy of the cerebellum, and 18F-fluorodeoxyglucose positron-emission tomography demonstrated glucose hypometabolism of the cerebellum.
Sjögren's syndrome is a chronic autoimmune disorder of unknown etiology and pathogenesis. It is characterized mainly by mononuclear cell infiltration of the exocrine glands, which leads to dryness of the eyes and mouth.1 Although the disorder has a strong predilection toward women (females : males, 9 : 1), both sexes and all ages may be affected.2 Approximately one-third of patients with primary Sjögren's syndrome (PSS) present with systemic manifestations,3 but the prevalence of neurologic manifestations in PSS remains controversial.4-6 Peripheral neuropathy is reportedly the most common neurologic complication of PSS, followed by cranial nerve neuropathy and focal or multifocal involvement of the central nervous system (CNS).4 Although ataxia due to PSS has also been described,7,8 marked cerebellar atrophy associated with PSS has rarely been reported.
We describe herein a patient with PSS who exhibited rapidly progressive ataxia accompanied by cerebellar atrophy.
A 46-year-old woman was admitted to our neurologic clinic due to progressive gait disturbance. Three months before admission, she developed vitiligo in her right anterior neck area, and she noticed unsteadiness of her gait. There was no family history of neurologic disorders and the patient had no exposure to toxins such as alcohol or drugs.
A neurological examination revealed that she had cerebellar dysfunction: dysarthria, nystagmus, and ataxia. Ataxia was observed in all four extremities, but was worse in the left limbs and trunk. The patient had marked gait disturbance due to ataxia. Speech analysis revealed that her speech disturbance was a mixed type of dysarthria with ataxic, hyperkinetic, and flaccid components. Gaze-evoked nystagmus of both eyes was found in all directions. Motor power and sensory examinations were normal. She had no parkinsonian features, her deep-tendon reflexes were normoactive, and her plantar responses were flexor bilaterally. She had mild depression and persecutory delusions related to her husband.
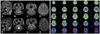
Laboratory tests were positive for speckled-type antinuclear antibody, anti-Ro/SSA (4.5 S/C ratio), and anti-La/SSB (1.0 S/C ratio). Her antinuclear antibody titer was elevated by 1 : 160. The following parameters were all normal: complete blood counts, biochemical screening, erythrocyte segmentation rate, C-reactive protein, thyroid function test, anti-hepatitis C virus, Venereal Disease Research Laboratory test, serum C3, C4, and CH50, anti-ds-DNA, lupus anticoagulant, anticardiolipin antibody, cytoplasmic antineutrophil cytoplasmic antibodies, perinuclear antineutrophil cytoplasmic antibodies, serum folate, vitamin B12, anti-human immunodeficiency virus (HIV) antibody, HIV antigen, and paraneoplastic antibodies (anti-Hu, anti-Ri, anti-Yo, and anti-CRMP5). The following malignancy screening procedures produced unremarkable results: whole-body 18F-fluorodeoxyglucose positron-emission tomography (18F-FDG PET), tumor markers (alpha fetoprotein, carcinoembryonic antigen, CA-125, CA-19-9, and CA 15-3), cerebrospinal fluid cytology, mammography, and abdomen-pelvic computed tomography scan. The findings of brain magnetic resonance imaging (MRI) were normal (Fig. 1A-D).
Her ataxia and gait disturbance progressively worsened over the next 6 months. She had mild cognitive impairment and scored 25 on the Korean version of the Mini Mental Status Examination. Her Montreal Cognitive Assessment9 score was 14. The patient exhibited impairment in frontal executive function, visuospatial function, attention, calculation, and delayed recall on bedside examination. Oculomotor examination revealed mild saccadic pursuit eye movements and saccadic dysmetria. Positional nystagmus of both eyes was purely horizontal, and was triggered by an apogeotropic head position, which was not associated with vertigo. The nystagmus was not improved by repetitive canalith-repositioning maneuvers. She suffered from moderate xerostomia and xerophthalmia.
Follow-up brain MRI revealed marked cerebellar atrophy with an enlarged fourth ventricle and cisterna magna (Fig. 1E-H). Brain 18F-FDG PET revealed decreased glucose metabolism in the bilateral cerebellum (Fig. 1I). Mutation analysis for dentatorubral-pallidoluysian atrophy and spinocerebellar ataxia types 1, 2, 3, 6, and 7 were negative. A salivary scan revealed no uptake of the parotid and submandibular glands (Fig. 2A). Biopsy of a minor salivary gland revealed focal lymphocytic infiltration involving exocrine glands (Fig. 2B). The result of Schirmer's test was strongly positive. The patient was diagnosed with PSS according to American and European criteria.10
Intravenous methylprednisolone (500 mg/day) was administered for 3 days and then maintenance therapy with oral methylprednisolone (1 mg/kg/day) was continued for 1 month. Hydroxychloroquine (200 mg, t.i.d.), quetiapine (12.5 mg/day), escitalopram (5 mg/day), and buspirone (10 mg, t.i.d.) were also administered. However, there was no improvement in her neurological and psychiatric abnormalities. Unfortunately, her gait disturbance and dysarthria progressed further, with persistent psychiatric symptoms during the 18-month follow-up period.
Primary cerebellar degeneration is extremely rarely associated with PSS. We evaluated possible causes of primary cerebellar degeneration in our patient, including alcohol, drug abuse, nutritional deficiency, genetic causes of cerebellar ataxia, paraneoplastic marker, autoimmune disorders, and a rare manifestation of HIV infection.11 Diagnosis by exclusion ultimately led to the belief that her cerebellar degeneration was related to PSS.
Our patient with established PSS exhibited rapidly progressive cerebellar ataxia and subacute development of cerebellar atrophy. In contrast to the uniform features of the peripheral nervous system complications of PSS, CNS abnormalities are associated with a much wider spectrum of manifestations.5 Our patient had severe pancerebellar dysfunction that did not respond to medical treatment.
The pathophysiologic mechanisms underlying the CNS involvement in PSS remain unclear. Previous studies have suggested that ischemia plays a role because CNS lesions in PSS were found to be associated with mononuclear inflammation and cerebral ischemic vasculopathy, resulting in micro or macro infarcts.6,12,13 Other studies found that abnormalities in humoral and cellular immunity, including antineuronal antibody, might be involved in the pathogenesis of the CNS manifestations of Sjögren's syndrome.8,13 In addition, the direct involvement of anti-Ro/SSA antibodies has been suggested.6 Interestingly, other autoimmune diseases, such as Behçet's disease and systemic lupus erythematosus, have also been reported to present with cerebellar atrophy (Table 1).
Our patient exhibited mild neuropsychiatric dysfunction. The most frequently observed CNS disorders in PSS are non-focal involvements such as memory loss, cognitive dysfunction, visual disturbance, dizziness, and reduced performance in concentration and attention.14 The incidence of mild-to-moderate psychiatric and cognitive dysfunction may be as high as 80% in patients with CNS manifestations in PSS.15 Psychiatric disorders are common in PSS, including atypical mood disorder, psychosis, paranoia, and depression, all of which were found in our patient.4,15 Cerebral white-matter hyperintensities on MRI, or cerebral hypoperfusion in the frontal, parietal, and temporal cortices in functional studies have been found to be correlated with mild cognitive dysfunction in PSS.15,16 A recent published evaluation of cognitive dysfunction in PSS described executive and visuospatial dysfunctions in PSS even in patients with no history of neurological involvement.16 However, in our case there were no cerebral parenchymal abnormalities, and cerebral glucose metabolism was preserved, suggesting that another pathophysiologic mechanism was responsible for her neuropsychiatric manifestation.
It is reported that the cerebellum modulates cognitive and affective functioning.17 The term "cerebellar cognitive affective syndrome" has been suggested to describe the disturbance of executive function, impaired spatial cognition, personality change, and linguistic difficulties.17 In addition, cognitive impairment and psychiatric symptoms, including depression, personality change, anxiety, and psychosis, were noted in 51% of patients with cerebellar degeneration.18 It was suggested that the cerebellar feedback loop through the thalamus to the cerebral cortex is directed not only to the sensorimotor cortices but also to the associative cerebrocerebellar circuitry, including the dorsolateral cortex, dorsomedial prefrontal cortex, parietal cortex, and limbic system.17 In our patient there was no evidence of supratentorial abnormalities on brain MRI or 18F-FDG PET. Therefore, the cognitive and neuropsychiatric manifestations of our patient may have been related to the functional disconnection of associative cerebellar circuits.
Positional nystagmus in our patient was one of the characteristic manifestations of marked cerebellar degeneration. Various diseases involving the fourth ventricle or cerebellar vermis, such as stroke, multiple sclerosis, multiple system atrophy, and tumor, have been reported to cause positional nystagmus.19-23 Paroxysmal direction-changing positional nystagmus has been also reported in patients with the horizontal canal variant of benign positional vertigo.24 Our patient had other oculomotor dysfunctions, including saccadic pursuit, hypermetric saccade, and gaze-evoked nystagmus without vertigo, and did not benefit from repetitive canalith-repositioning maneuvers, suggesting positional nystagmus with a central origin.
In summary, the reported PSS patient exhibited a progressive course of cerebellar syndrome, which was demonstrated by cerebellar atrophy on serial MRI and 18F-FDG PET. Further experimental studies are needed to clarify the precise pathogenic mechanism underlying marked cerebellar degeneration in PSS.
Figures and Tables
Fig. 1
Brain MRI and 18F-FDG PET results for the patient. A-D: Initial brain MRI was unremarkable. E-H: Follow-up brain MRI, performed 6 months later, revealed marked cerebellar atrophy and an enlarged fourth ventricle and cisterna magna without cerebral cortical atrophy. I: 18F-FDG PET, performed at the same time as the follow-up MRI, revealed decreased glucose metabolism in the bilateral cerebellum. 18F-FDG PET: 18F-fluorodeoxyglucose positron-emission tomography.
References
1. Ramos-Casals M, Font J. Primary Sjögren's syndrome: current and emergent aetiopathogenic concepts. Rheumatology (Oxford). 2005. 44:1354–1367.
2. Manoussakis MN, Moutsopoulos HM. Sjögren's syndrome: current concepts. Adv Intern Med. 2001. 47:191–217.
3. Skopouli FN, Dafni U, Ioannidis JP, Moutsopoulos HM. Clinical evolution, and morbidity and mortality of primary Sjögren's syndrome. Semin Arthritis Rheum. 2000. 29:296–304.

4. Delalande S, de Seze J, Fauchais AL, Hachulla E, Stojkovic T, Ferriby D, et al. Neurologic manifestations in primary Sjögren syndrome: a study of 82 patients. Medicine (Baltimore). 2004. 83:280–291.
5. Mauch E, Völk C, Kratzsch G, Krapf H, Kornhuber HH, Laufen H, et al. Neurological and neuropsychiatric dysfunction in primary Sjögren's syndrome. Acta Neurol Scand. 1994. 89:31–35.

6. Alexander E. Central nervous system disease in Sjögren's syndrome. New insights into immunopathogenesis. Rheum Dis Clin North Am. 1992. 18:637–672.
7. Wong S, Pollock AN, Burnham JM, Sherry DD, Dlugos DJ. Acute cerebellar ataxia due to Sjögren syndrome. Neurology. 2004. 62:2332–2333.
8. Owada K, Uchihara T, Ishida K, Mizusawa H, Watabiki S, Tsuchiya K. Motor weakness and cerebellar ataxia in Sjögren syndrome--identification of antineuronal antibody: a case report. J Neurol Sci. 2002. 197:79–84.

9. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005. 53:695–699.

10. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002. 61:554–558.

11. Tagliati M, Simpson D, Morgello S, Clifford D, Schwartz RL, Berger JR. Cerebellar degeneration associated with human immunodeficiency virus infection. Neurology. 1998. 50:244–251.

12. Alexander EL. Central nervous system (CNS) manifestations of primary Sjögren's syndrome: an overview. Scand J Rheumatol Suppl. 1986. 61:161–165.
13. Alexander EL, Lijewski JE, Jerdan MS, Alexander GE. Evidence of an immunopathogenic basis for central nervous system disease in primary Sjögren's syndrome. Arthritis Rheum. 1986. 29:1223–1231.

14. Massara A, Bonazza S, Castellino G, Caniatti L, Trotta F, Borrelli M, et al. Central nervous system involvement in Sjögren's syndrome: unusual, but not unremarkable--clinical, serological characteristics and outcomes in a large cohort of Italian patients. Rheumatology (Oxford). 2010. 49:1540–1549.

15. Cox PD, Hales RE. CNS Sjögren's syndrome: an underrecognized and underappreciated neuropsychiatric disorder. J Neuropsychiatry Clin Neurosci. 1999. 11:241–247.
16. Le Guern V, Belin C, Henegar C, Moroni C, Maillet D, Lacau C, et al. Cognitive function and 99mTc-ECD brain SPECT are significantly correlated in patients with primary Sjogren syndrome: a case-control study. Ann Rheum Dis. 2010. 69:132–137.

17. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998. 121:561–579.

18. Liszewski CM, O'Hearn E, Leroi I, Gourley L, Ross CA, Margolis RL. Cognitive impairment and psychiatric symptoms in 133 patients with diseases associated with cerebellar degeneration. J Neuropsychiatry Clin Neurosci. 2004. 16:109–112.

19. Johkura K. Central paroxysmal positional vertigo: isolated dizziness caused by small cerebellar hemorrhage. Stroke. 2007. 38:e26–e27. author reply e28.

20. Bertholon P, Antoine JC, Martin C, Michel D. Simultaneous occurrence of a central and a peripheral positional nystagmus during the Dix-Hallpike manoeuvre. Eur Neurol. 2003. 50:249–250.

21. Katsarkas A. Positional nystagmus of the "central type" as an early sign of multiple sclerosis. J Otolaryngol. 1982. 11:91–93.
22. Kattah JC, Kolsky MP, Luessenhop AJ. Positional vertigo and the cerebellar vermis. Neurology. 1984. 34:527–529.

23. Gregorius FK, Crandall PH, Baloh RW. Positional vertigo with cerebellar astrocytoma. Surg Neurol. 1976. 6:283–286.
24. Baloh RW, Jacobson K, Honrubia V. Horizontal semicircular canal variant of benign positional vertigo. Neurology. 1993. 43:2542–2549.

25. Terao Y, Sakai K, Kato S, Tanabe H, Ishida K, Tsukamoto T. Antineuronal antibody in Sjögren's syndrome masquerading as paraneoplastic cerebellar degeneration. Lancet. 1994. 343:790.

26. Manto MU, Rondeaux P, Jacquy J, Hildebrand JG. Subacute pancerebellar syndrome associated with systemic lupus erythematosus. Clin Neurol Neurosurg. 1996. 98:157–160.

27. Shimomura T, Kuno N, Takenaka T, Maeda M, Takahashi K. Purkinje cell antibody in lupus ataxia. Lancet. 1993. 342:375–376.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download