Abstract
Background and Purpose
The coexistence of carotid atherosclerosis in ischemic stroke patients with small-vessel disease (SVD) or intracranial large-vessel disease (ICLVD) was investigated using carotid duplex ultrasonography, and whether its coexistence affected the clinical prognosis was determined.
Methods
Ischemic stroke patients with SVD or ICLVD were enrolled (n=103). Risk factors, demographic data, and National Institutes of Health Stroke Scale (NIHSS) scores were obtained for all of the subjects. Early neurological progression was defined by an increase in NIHSS score during the first 7 days. Carotid ultrasonography was performed to measure the intima-media thickness (IMT) and carotid plaques.
Results
Among the 103 patients who were retrospectively enrolled in this study (56 with SVD and 47 with ICLVD), 66 (64.1%) had an atherosclerotic plaque and 23 (22.3%) had increased IMT. Increased IMT was observed more frequently in ICLVD than in SVD [15/47 (31.9%) vs. 8/56 (14.3%), p=0.032]. An atherosclerotic plaque was observed on subsequent carotid ultrasonographic examination in 28 (50%) of the 56 patients whose computed tomography angiography scans of the neck vessels were interpreted as normal. There was no association between presence of atherosclerotic change and early neurologic progression (p=0.94).
Ischemic stroke can be subdivided into four subtypes: small-vessel disease (SVD), intracranial large-vessel disease (ICLVD), extracranial large-vessel disease, and cardioembolism.1 However, since these subtypes have several risk factors in common that largely contribute to the development of atherosclerosis, carotid atherosclerosis may often coexist even with SVD and ICLVD, in which significant extracranial carotid atherosclerosis is considered to be absent using current diagnostic tools such as magnetic resonance (MR) angiography or computed tomography (CT) angiography.1 However, noninvasive ultrasonographic examinations of the intima-media thickness (IMT) and carotid plaques can provide precise information regarding early or minimal atherosclerotic change in stroke patients before it advances.2 Comprehensive sonographic examinations of the carotid artery are not routinely performed in acute stroke evaluation unless CT or MR angiography reveals a significant stenosis, which has resulted in little being known about how commonly carotid atherosclerotic change coexists with SVD and ICLVD and, when it does, how severe those changes are and the clinical implications of this coexistence.
The aim of this study was therefore to characterize the carotid IMT and plaques in patients with SVD and ICLVD using carotid duplex ultrasonography, and to determine whether the coexistence of carotid atherosclerosis with these two conditions affects the clinical prognosis.
We retrospectively included 103 consecutive acute ischemic stroke patients within 7 days of stroke onset who had been admitted to Yeouido St. Mary's Hospital and whose final diagnosis was either SVD or ICLVD according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification.1 Neurologic examinations, electrocardiography, routine blood tests with lipid profile, chest radiography, CT angiography (n=93) or MR angiography (n=10), carotid duplex ultrasonography, and MR imaging were performed. Transthoracic echocardiography and 24-hour electrocardiography monitoring were also applied to patients who were suspected to have cardioembolic stroke. The demographic data, initial National Institutes of Health Stroke Scale (NIHSS) score, stroke subtype according to the TOAST classification, and risk factors (hypertension, hyperlipidemia, diabetes, and smoking) were obtained for each patient. Hypertension was defined for a systolic blood pressure of ≥140 mm Hg and/or a diastolic blood pressure of ≥90 mm Hg, or treatment with antihypertensive medication. Diabetes mellitus was defined as a fasting plasma glucose level of ≥126 mg/dL, a random plasma glucose level of ≥200 mg/dL, an HbA1c level of ≥6.5%, or the reported use of a treatment for diabetes mellitus. Early neurological progression was defined by any increase in NIHSS score during the first 7 days of admission.
SVD was defined as the presence of a supratentorial lesion of less than 20 mm or a 15-mm infratentorial infarcted lesion without other probable source such as cardioembolism or significant proximal large-vessel disease. ICLVD was defined as the presence of an ischemic infarction resulting from occlusion of the intracranial arteries without proximal arterial disease or cardioembolism.
This study was approved by the Institutional Review Board of our institution. The patient's informed consent to participate was not obtained, and was not required because this study was performed as a retrospective review.
Helical [three-dimensional (3D)] CT angiography was performed with a LightSpeed VCT system (GE Medical Systems, Milwaukee, WI, USA). 3D CT angiography was performed with a computer workstation (Rapidia, Infinitt, Seoul, Korea). The MR angiography (CVi, GE Medical Systems) parameters included a flip angle of 20°, a 320×224 matrix, and a field-of-view of 200×200. 3D contrast-enhanced MR angiography scans were obtained from the aortic arch to the level of the central skull base with an intravenous, 15-mL bolus injection of gadopentetate dimeglumine, a repetition time of 27 ms, and an echo time of 6.9 ms.
Duplex ultrasonography was performed using a General Electronics LOGIQ 7.0 system (GE Medical Systems) to measure IMT and carotid plaques.3 IMT was measured at the far wall of each common carotid artery. A region 1 cm proximal to the bulb was first identified. The measurement was made at three continuous sites separated by approximately 1 cm. If IMT was increased at both sides, we chose the thicker measurement. 'Increased IMT' was defined when the average IMT was >1 mm. Plaques were defined as focal structures that encroached upon the arterial lumen by at least 0.5 mm or 50% of the surrounding IMT. When a plaque was present, IMT was measured at the nearest plaque-free point. All sonograms were evaluated independently by one investigator (H.-Y. Jee). All of the cases were reviewed by one stroke neurologist (A.-H. Cho) immediately after acquisition, who was blinded to the clinical characteristics as listed previously.
Between November 2007 and May 2009, the data of 103 patients were obtained for analysis (56 with SVD and 47 with ICLVD). Among them, 66 (64.1%) patients had an atherosclerotic plaque and 23 (22.3%) had increased IMT. Any atherosclerotic change (presence of a plaque or increased IMT) was observed in 74 patients (Fig. 1). A plaque was observed slightly more commonly in ICLVD than in SVD [31/47 (66.0%) vs. 35/56 (62.5%)], but the difference was not significant (p=0.71). The plaque thickness was 2.7±1.1 mm (mean±SD, range 1.5-8.0 mm). An increased IMT was observed more frequently in ICLVD than in SVD [15/47 (31.9%) vs. 8/56 (14.3%), p=0.032; case 3, Fig. 1J]. The risk factors did not differ between SVD and ICLVD (Table 1).
Risk factors and initial NIHSS scores did not differ between patients with and without a carotid plaque. However, patients with a carotid plaque were significantly older (67.5±11.4 years vs. 59.6±11.29 years; p=0.002) (Table 2). Plaques were categorized into five types: 1) echogenic (n=25 patients), 2) isoechoic (n=14), 3) echolucent (n=12), 4) mixed echogenicity (n=11), and 5) unclassified (n=4).
Regarding the correlation between CT angiographic findings and duplex findings (cases 1, 2 and 3; Fig. 1B, E and I, respectively), atherosclerotic plaque on duplex sonographic examinations were observed in 28 of 56 patients (50%) who were regarded as normal according to the CT angiography (e.g., case 1, Fig. 1B and C; case 2, Fig. 1E and F; case 3, Fig. 1I and J). Early neurological progression was observed in 16 patients, of whom a plaque was observed in 10. There was no correlation between carotid artery atherosclerotic change and early neurologic progression in the acute stroke patients [10/16 (62.5%) vs. 45/73 (61.6%), p=0.94).
This is the first study to investigate carotid artery status in ischemic stroke patients with duplex ultrasonography, and to compare this with CT or MR angiographic data. A similar previous study found that a higher IMT was more likely to be associated with nonlacunar stroke than with lacunar stroke,4 and the results of another study that evaluated IMT and plaque scores in stroke patients suggested a relationship between atherothrombotic infarction and these two parameters.5 However, those studies did not perform CT or MR angiographic examinations in all patients. In our study, 71.8% of patients with SVD or ICLVD (according to the TOAST classification) had coexisting carotid atherosclerotic changes on duplex ultrasonography. In addition, CT or MR angiographic findings underestimated the presence of a carotid plaque in 50% of patients in whom the angiographic results were interpreted as normal patent vessels. This is probably attributable to the methodological characteristics of CT and MR angiography, such as a flow artifacts and visualization of the artery being restricted to its intraluminal appearance, which can result in significant but longitudinally located plaques with smooth surfaces easily being overlooked. However, such plaques require long-term treatment such as statin use, risk factor modification, and serial follow-ups.
Stroke subtype classification is performed in order to clearly define and divide the stroke etiology, but the findings of our study suggest that a clear-cut pattern of one stroke subtype is uncommon. Rather, the coexistence of carotid atherosclerosis with SVD or intracranial artery disease may be more prevalent. Each stroke victim seems to be at a certain point in the clinical spectrum from SVD to intracranial or extracranial large-artery atherosclerosis because all such victims share similar risk factors and prevention strategies. Although the coexistence of an atherosclerotic plaque presents only a small burden, echolucent plaques with irregular ulcerative surfaces are a possible source of thromboembolism leading to another stroke that will require active medical management. Therefore, a comprehensive assessment of carotid status through noninvasive ultrasonography would be useful for identifying carotid plaques or IMT, which can be underestimated by CT or MR angiography.
In the present study, increased IMT was more common in patients with ICLVD than in those with SVD. This finding appears to support the atherosclerotic pathogenesis of intracranial arterial stenosis. However, there was no difference between ICLVD and SVD regarding the incidence of carotid plaques. Although IMT and plaques are strongly correlated, they may reflect different biological aspects of atherogenesis.6 A correlation between increased IMT and presence of a carotid plaque was not found in a previous related study.7 Moreover, no significant relationship was found for the contribution of coexisting carotid atherosclerosis to early neurological progression in the present study.
There are several limitations to this study. The small sample necessitates prudent interpretation of our data. Furthermore, statin use was not detailed in our results; however, the prevalence of hyperlipidemia did not differ between SVD and ICLVD. Thus, we do not believe that the results of our descriptive study were affected by statin use. Finally, we used the common carotid artery rather than the internal carotid artery for IMT measurement, and the IMT in these two arteries may involve different biological aspects, which may lead to different results.
Figures and Tables
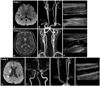
Fig. 1
Case 1: A 43-year-old male patient presented with lacunar infarction on the right basal ganglia (A). The carotid artery appeared to be patent on neck computed tomography (CT) angiography (B). However, ultrasonography revealed an increased intima-media thickness (IMT) and a focal plaque on the left common carotid artery (C). Case 2: A 54-year-old male patient with a left basal ganglia infarction had a focal atherosclerotic plaque on the left bulb (D and F), which was not detected on CT angiography (E). Case 3: A 58-year-old man with a right middle cerebral artery (MCA) territorial infarction from a right MCA stenosis appeared to have a patent carotid artery (G-I). However, a diffusely increased IMT was observed on ultrasonographic examination (J).
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (No. 2010-0002537).
References
1. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993. 24:35–41.

2. Nagai Y, Matsumoto M, Metter EJ. The carotid artery as a noninvasive window for cardiovascular risk in apparently healthy individuals. Ultrasound Med Biol. 2002. 28:1231–1238.

3. Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007. 23:75–80.

4. Cupini LM, Pasqualetti P, Diomedi M, Vernieri F, Silvestrini M, Rizzato B, et al. Carotid artery intima-media thickness and lacunar versus nonlacunar infarcts. Stroke. 2002. 33:689–694.

5. Nagai Y, Kitagawa K, Yamagami H, Kondo K, Hougaku H, Hori M, et al. Carotid artery intima-media thickness and plaque score for the risk assessment of stroke subtypes. Ultrasound Med Biol. 2002. 28:1239–1243.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download