Abstract
Background and Purpose
The aim of this study was to elucidate the clinical features, prothrombotic risk factors, and outcome of pediatric Moyamoya patients.
Methods
Patients diagnosed with Moyamoya disease at a tertiary center between January 2000 and December 2006 were enrolled in this study. The clinical presentations, underlying diseases, prothrombotic risk factors, family history of thrombosis, radiological findings, treatment, and outcome of the patients were reviewed retrospectively.
Results
Eight patients with angiographically proven Moyamoya disease were identified, one of whom had neurofibromatosis type I and one had Down syndrome. The age at diagnosis varied between 19 months and 11 years (73.4±41.8 months, mean±SD). The follow-up period after diagnosis was 52.5±14.8 months. In six patients, the initial clinical presentation was hemiparesis. None of the patients had any identifiable prothrombotic factors. Despite medical and surgical treatment, three patients had recurrences and one died. Only two patients recovered without sequelae.
Moyamoya disease is a rare and chronic cerebrovascular occlusive disorder that is characterized by a progressive stenosis or obstruction of the internal carotid artery and proximal cerebral arteries, with development of an extensive network of cerebral collaterals.1 The etiology of the disease remains unclear; higher incidences in Japan and Korea point to a possible genetic mode of inheritance. Familial occurrence has also been reported among the Japanese and Caucasian populations.2,3 The clinical features of the disease are cerebral ischemia, recurrent transient ischemic attacks, sensorimotor paralysis, and seizures. Moyamoya disease generally presents with repeated ischemic stroke in children and intracranial hemorrhage in adults.4
The aim of this study was to review the clinical features, prothrombotic risk factors, and outcome of Moyamoya patients diagnosed at our Pediatric Neurology Department, with a view to improving the understanding of this disease.
Patients diagnosed with Moyamoya disease between January 2000 and December 2006 were enrolled in the study. The following parameters were reviewed retrospectively: clinical presentations, underlying diseases, prothrombotic risk factors (erythrocyte sedimentation rate, C-reactive protein, complete blood count, renal and hepatic chemistry, lipid profile, ammonia, lactate and homocysteine levels, prothrombin time, partial thromboplastin time, activated partial thromboplastin time, fibrinogen, protein C and protein S levels, antithrombin III and coagulation factor levels, lupus anticoagulant and anticardiolipin antibodies, factor V Leiden mutation, prothrombin 20210 gene mutation, and methylenetetrahydrofolate reductase gene mutation), family history of thrombosis, radiological findings, treatment, and outcome of the patients. Prothrombotic risk factors were evaluated twice, 3-6 months apart. The ranges of normal values were adjusted relative to age. All analyses were carried out at the same pediatric biochemistry, hematology, and metabolism laboratories of Istanbul University Hospital.
Eight patients (four male, four female) were diagnosed with Moyamoya disease between 2000 and 2006. The age at diagnosis varied between 19 months and 11 years (73.4±41.8 months, mean±SD). The follow-up period after diagnosis was 52.5±14.8 months. One of the patients had neurofibromatosis type I (Fig. 1) and one had Down syndrome. All patients had bilateral stenosis or occlusion of the distal internal carotid arteries associated with an abnormal network of fine collateral vessels at the base of the brain (Figs. 2 and 3). One patient also had left anterior cerebral artery occlusion, one had bilateral proximal middle cerebral artery stenosis, and two had left proximal middle cerebral artery stenosis (Table 1).
The initial clinical presentation in six patients was hemiparesis, which was accompanied by focal seizures in patient 2 and by aphasia in patient 4. Hemiparesis alternated from side to side between recurrences in patient 6. One patient presented only with a severe migraine-like headache.
Prothrombotic disorders were evaluated in all patients. There were no relevant family histories and none of the patients had prothombotic risk factors, but the final conclusion was only reached after the tests were repeated 3-6 months after the initial diagnosis.
Four patients underwent surgical revascularization procedures, two of which were bilateral. Each of these four patients underwent encephaloduroarteriosynangiosis. Follow-up information on disability and functional status was available for all of the patients. One patient died (Fig. 4) and three had recurrent strokes (patient 1 also experienced strokes after surgery). Of the seven remaining patients, two had no disability (modified Rankin Scale score of 0 or 1) and five had mild or moderate disability but were able to walk (modified Rankin Scale score of 2 or 3).
Pediatric patients with Moyamoya disease are known to present with transient ischemic attacks or ischemic strokes, whereas adult patients tend to suffer from intracranial bleeding. Involuntary choreiform movements, seizures, and migraine-like headaches can also be the initial sign of the disease. Some patients may be identified incidentally.1,4 Hemiparesis was the most common clinical presentation in our cohort, but its alternating nature can lead to diagnostic challenges. A high index of suspicion is mandatory for diagnosis in patients with headaches and genetic syndromes.
Inherited and acquired prothrombotic disorders have been identified as the cause of stroke in young people.5 Several reports have described the probable connection between hemostatic abnormalities and Moyamoya disease.6-8 Two of these are single case reports of patients with protein S and protein C and S deficiencies.6,8 A prospective study of ten consecutive pediatric Moyamoya patients by Bonduel et al.6 revealed inherited protein S deficiency in one patient and lupus anticoagulant and anticardiolipin antibodies in three patients. In contrast to these reports, none of our patients had any identifiable prothrombotic risk factors. The paucity of single case reports represents evidence for the rarity of coincidence between Moyamoya disease and prothrombotic disorders.
Conditions such as sickle cell anemia, neurofibromatosis type 1, Down syndrome, congenital heart defects, antiphospholipid syndrome, renal artery stenosis, and thyroiditis have been found to be associated with Moyamoya disease in the medical literature.9-11 Since one of our patients had neurofibromatosis type I and one had Down syndrome, the presence of Moyamoya disease should be considered in the evaluation of patients who present with neurological symptoms.
Moyamoya disease typically follows a progressive course with motor and intellectual deterioration, and rarely remains stable.11 Few patients recover without sequelae. When the occlusive process halts and the maximum number of collateral vessels has developed, the clinical course stabilizes. There is growing evidence for the theory that surgery is beneficial in reducing ischemic symptoms and improving the neurological outcome. Some authors believe that the outcome is better after surgery than after medical treatment or the natural course of the disease.12-14 The prognosis in children is worse when the onset of symptoms occurs at a younger age.15 Our outcomes were in accordance with this pattern; however, we were unable to detect any difference in functional outcome between medical and surgical patients overall.
While there were too few patients in our study to enable a definitive conclusion to be drawn about the link between prothrombotic risk factors and Moyamoya disease, thrombotic risk factors are generally rarely reported in patients with Moyamoya disease. Thus, the value of prothrombotic risk factor evaluation in these patients seems to be limited. The rarity of this disease necessitates multicenter studies to further develop an understanding of the underlying mechanisms and to determine the most appropriate approach for the treatment of pediatric Moyamoya patients.
Figures and Tables
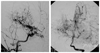 | Fig. 1Angiography of the patient with Neurofibromatosis type 1, displaying characteristic puff of the smoke sign. |
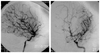 | Fig. 3Angiography of 7 years old female patient, displaying occlusion of middle cerebral arteries and formation of collaterals. |
References
1. Gosalakkal JA. Moyamoya disease: a review. Neurol India. 2002. 50:6–10.
2. Ikezaki K, Han DH, Kawano T, Kinukawa N, Fukui M. A clinical comparison of definite moyamoya disease between South Korea and Japan. Stroke. 1997. 28:2513–2517.

3. Shetty-Alva N, Alva S. Familial moyamoya disease in Caucasians. Pediatr Neurol. 2000. 23:445–447.

5. deVeber G, Monagle P, Chan A, MacGregor D, Curtis R, Lee S, et al. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch Neurol. 1998. 55:1539–1543.

6. Bonduel M, Hepner M, Sciuccati G, Torres AF, Tenembaum S. Prothrombotic disorders in children with moyamoya syndrome. Stroke. 2001. 32:1786–1792.

7. Akgün D, YiLmaz S, Senbil N, Aslan B, Gürer YY. Moyamoya syndrome with protein S deficiency. Eur J Paediatr Neurol. 2000. 4:185–188.

8. Cheong PL, Lee WT, Liu HM, Lin KH. Moyamoya syndrome with inherited proteins C and S deficiency: report of one case. Acta Paediatr Taiwan. 2005. 46:31–34.
9. Lutterman J, Scott M, Nass R, Geva T. Moyamoya syndrome associated with congenital heart disease. Pediatrics. 1998. 101:57–60.

10. Jea A, Smith ER, Robertson R, Scott RM. Moyamoya syndrome associated with Down syndrome: outcome after surgical revascularization. Pediatrics. 2005. 116:e694–e701.

11. Hogan AM, Kirkham FJ, Isaacs EB, Wade AM, Vargha-Khadem F. Intellectual decline in children with moyamoya and sickle cell anaemia. Dev Med Child Neurol. 2005. 47:824–829.

12. Scott RM. Surgery for moyamoya syndrome? Yes. Arch Neurol. 2001. 58:128–129.
13. Golby AJ, Marks MP, Thompson RC, Steinberg GK. Direct and combined revascularization in pediatric moyamoya disease. Neurosurgery. 1999. 45:50–58.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download