1. Dumitru D, Zwartz MJ. Dumitru D, Amato AA, Zwarts M, editors. Electrodiagnostic medicine pitfalls. Electrodiagnostic Medicine. 2001. 2nd ed. Philadelphia: Hanley & Belfus;p. 541–577.

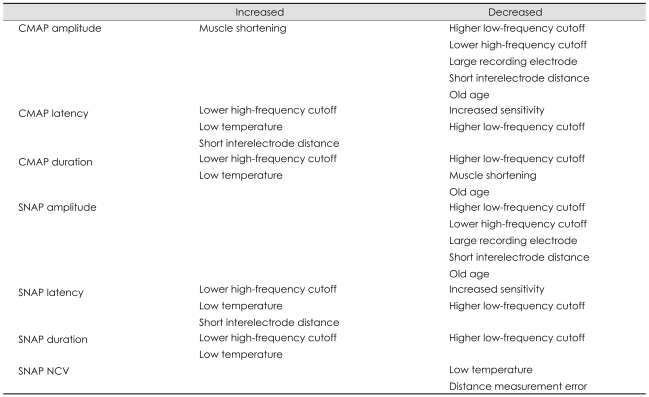
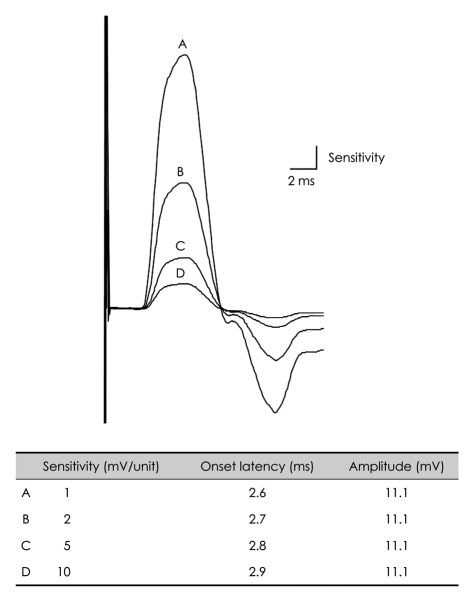
2. Goldfarb AR, Saadeh PB, Sander HW. Effect of amplifier gain setting on distal motor latency in normal subjects and CTS patients. Clin Neurophysiol. 2005; 116:1581–1584. PMID:
15905123.

3. Dumitru D, Zwartz MJ. Dumitru D, Amato AA, Zwarts M, editors. Instrumentation. Electrodiagnostic Medicine. 2001. 2nd ed. Philadelphia: Hanley & Belfus;p. 69–97.
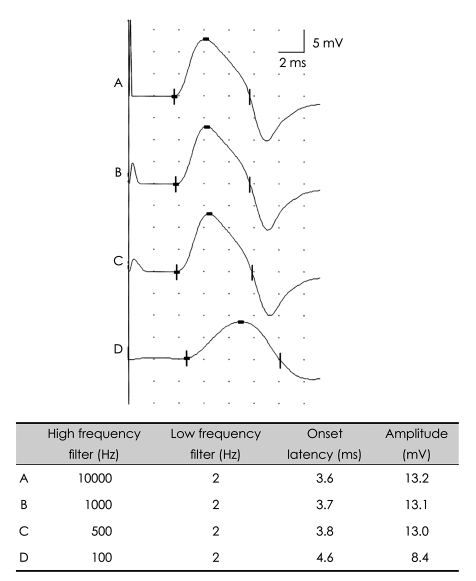
4. Dumitru D, Walsh NE. Practical instrumentation and common sources of error. Am J Phys Med Rehabil. 1988; 67:55–65. PMID:
3355677.

5. Oh SJ. Oh SJ, editor. Basic components of electromyography instruments. Clinical Electromyography: Nerve Conduction Studies. 2002. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;p. 25–36.
6. van Dijk JG, Tjon-a-Tsien A, van der Kamp W. CMAP variability as a function of electrode site and size. Muscle Nerve. 1995; 18:68–73. PMID:
7800000.

7. Lee EJ, Baek DH, Baek JY, Kim BJ, Choi J, Pak JJ, et al. PDMS- and silver-ball-based flexible multichannel surface electrode: fabrication and application in nerve conduction study on patients with diabetic polyneuropathy. IEEE Sens J. 2009; 9:625–632.

8. Ven AA, Van Hees JG, Stappaerts KH. Effect of size and pressure of surface recording electrodes on amplitude of sensory nerve action potentials. Muscle Nerve. 2004; 30:234–238. PMID:
15266641.

9. Drenthen J, Blok JH, van Heel EB, Visser GH. Limb temperature and nerve conduction velocity during warming with hot water blankets. J Clin Neurophysiol. 2008; 25:104–110. PMID:
18340275.

10. Dumitru D, Amato AA, Zwartz MJ. Dumitru D, Amato AA, Zwarts M, editors. Nerve conduction studies. Electrodiagnostic Medicine. 2001. 2nd ed. Philadelphia: Hanley & Belfus;p. 159–223.

11. Eduardo E, Burke D. The optimal recording electrode configuration for compound sensory action potentials. J Neurol Neurosurg Psychiatry. 1988; 51:684–687. PMID:
2841424.

12. Lee HJ, DeLisa JA, Bach JR. Physiologic considerations in the determination of optimum interelectrode distance for the antidromic recording of compound sensory nerve action potentials. Commentary. Am J Phys Med Rehabil. 1993; 72:99–100. PMID:
8476551.
13. Oh SJ. Oh SJ, editor. Nerve conduction techniques. Clinical Electromyography: Nerve Conduction Studies. 2002. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;p. 37–53.
14. Varghese G, Rogoff JB. Influence of inter-electrode distance on antidromic sensory potentials. Electromyogr Clin Neurophysiol. 1983; 23:297–301. PMID:
6872929.
15. Evanoff V Jr, Buschbacher RM. Optimal interelectrode distance in sensory and mixed compound nerve action potentials: 3- versus 4-centimeter bar electrodes. Arch Phys Med Rehabil. 2004; 85:405–408. PMID:
15031825.

16. Brashear A, Kincaid JC. The influence of the reference electrode on CMAP configuration: leg nerve observations and an alternative reference site. Muscle Nerve. 1996; 19:63–67. PMID:
8538671.

17. Kincaid JC, Brashear A, Markand ON. The influence of the reference electrode on CMAP configuration. Muscle Nerve. 1993; 16:392–396. PMID:
8455652.

18. Nandedkar SD, Barkhaus PE. Contribution of reference electrode to the compound muscle action potential. Muscle Nerve. 2007; 36:87–92. PMID:
17455266.
19. Kim BJ, Date ES, Lee SH, Yoon JS, Hur SY, Kim SJ. Distance measure error induced by displacement of the ulnar nerve when the elbow is flexed. Arch Phys Med Rehabil. 2005; 86:809–812. PMID:
15827936.

20. Okamoto M, Abe M, Shirai H, Ueda N. Morphology and dynamics of the ulnar nerve in the cubital tunnel. Observation by ultrasonography. J Hand Surg Br. 2000; 25:85–89. PMID:
10763732.
21. Childress HM. Recurrent ulnar-nerve dislocation at the elbow. Clin Orthop Relat Res. 1975; 168–173. PMID:
1139823.

22. Grevsten S, Lindsjö U, Olerud S. Recurrent ulnar nerve dislocation at the elbow. Report of a non-traumatic case with ulnar entrapment neuropathy. Acta Orthop Scand. 1978; 49:151–153. PMID:
676698.
23. Jacobson JA, Jebson PJ, Jeffers AW, Fessell DP, Hayes CW. Ulnar nerve dislocation and snapping triceps syndrome: diagnosis with dynamic sonography--report of three cases. Radiology. 2001; 220:601–605. PMID:
11526255.

24. Kim BJ, Koh SB, Park KW, Kim SJ, Yoon JS. Pearls & Oy-sters:false positives in short-segment nerve conduction studies due to ulnar nerve dislocation. Neurology. 2008; 70:e9–e13. PMID:
18195259.
25. Kim BJ, Date ES, Park BK, Choi BY, Lee SH. Physiologic changes of compound muscle action potentials related to voluntary contraction and muscle length in carpal tunnel syndrome. J Electromyogr Kinesiol. 2005; 15:275–281. PMID:
15763674.

26. Mori I, Hasegawa O. [Effect of posture and postural fixation in repetitive nerve stimulation test]. No To Shinkei. 1999; 51:867–870. PMID:
10553587.
27. Kornfield MJ, Cerra J, Simons DG. Stimulus artifact reduction in nerve conduction. Arch Phys Med Rehabil. 1985; 66:232–235. PMID:
3985775.

28. Tracey BH, Krishnamachari S. Automated removal of stimulus artifact in nerve conduction studies. Conf Proc IEEE Eng Med Biol Soc. 2006; 1:6360–6363. PMID:
17945961.

29. Dreyer SJ, Dumitru D, King JC. Anodal block V anodal stimulation. Fact or fiction. Am J Phys Med Rehabil. 1993; 72:10–18. PMID:
8431262.
30. Aprile I, Stålberg E, Tonali P, Padua L. Double peak sensory responses at submaximal stimulation. Clin Neurophysiol. 2003; 114:256–262. PMID:
12559232.

31. Yasunami T, Miyawaki Y, Kitano K, Okuno H. Shortening of distal motor latency in anode distal stimulation. Clin Neurophysiol. 2005; 116:1355–1361. PMID:
15978497.

32. Aprile I, Tonali P, Stalberg E, Di Stasio E, Caliandro P, Foschini M, et al. Double peak sensory responses: effects of capsaicin. Neurol Sci. 2007; 28:264–269. PMID:
17972041.

33. Dumitru D, Zwartz MJ. Dumitru D, Amato AA, Zwarts M, editors. Special nerve conduction techniques. Electrodiagnostic Medicine. 2001. 2nd ed. Philadelphia: Hanley & Belfus;p. 225–256.

34. Zehr EP. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol. 2002; 86:455–468. PMID:
11944092.

35. Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods. 2008; 171:1–12. PMID:
18394711.

36. Somjen G, Carpenter DO, Henneman E. Responses of motoneurons of different sizes to graded stimulation of supraspinal centers of the brain. J Neurophysiol. 1965; 28:958–965. PMID:
5867887.

37. Henneman E, Somjen G, Carpenter DO. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965; 28:599–620. PMID:
5835487.
38. Awiszus F, Feistner H. The relationship between estimates of Ia-EPSP amplitude and conduction velocity in human soleus motoneurons. Exp Brain Res. 1993; 95:365–370. PMID:
8224062.

39. Hale BS, Raglin JS, Koceja DM. Effect of mental imagery of a motor task on the Hoffmann reflex. Behav Brain Res. 2003; 142:81–87. PMID:
12798268.

40. Kiers L, Fernando B, Tomkins D. Facilitatory effect of thinking about movement on magnetic motor-evoked potentials. Electroencephalogr Clin Neurophysiol. 1997; 105:262–268. PMID:
9284233.

41. Takahashi N, Takahashi O, Ogawa S, Takahashi M. What is the origin of the premotor potential recorded from the second lumbrical? Muscle Nerve. 2006; 34:779–781. PMID:
16810692.

42. Johnsen B, Fuglsang-Frederiksen A, de Carvalho M, Labarre-Vila A, Nix W, Schofield I. Amplitude, area and duration of the compound muscle action potential change in different ways over the length of the ulnar nerve. Clin Neurophysiol. 2006; 117:2085–2092. PMID:
16876477.

43. Colachis SC 3rd, Klejka JP, Shamir DY, Pease WS, Johnson EW. Amplitude of M responses. Side to side comparability. Am J Phys Med Rehabil. 1993; 72:19–22. PMID:
8381651.

44. Tong HC, Werner RA, Franzblau A. Effect of aging on sensory nerve conduction study parameters. Muscle Nerve. 2004; 29:716–720. PMID:
15116376.

45. Shehab DK, Khuraibet AJ, Butinar D, Abraham MP, Jabre JF. Effect of gender on orthodromic sensory nerve action potential amplitude. Am J Phys Med Rehabil. 2001; 80:718–720. PMID:
11562552.

46. Fujimaki Y, Kuwabara S, Sato Y, Isose S, Shibuya K, Sekiguchi Y, et al. The effects of age, gender, and body mass index on amplitude of sensory nerve action potentials: multivariate analyses. Clin Neurophysiol. 2009; 120:1683–1686. PMID:
19640782.

47. Lacomis D. Small-fiber neuropathy. Muscle Nerve. 2002; 26:173–188. PMID:
12210380.

48. Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003; 13:256–270. PMID:
12955550.
49. Tobin K, Giuliani MJ, Lacomis D. Comparison of different modalities for detection of small fiber neuropathy. Clin Neurophysiol. 1999; 110:1909–1912. PMID:
10576486.

50. Rossi P, Serrao M, Amabile G, Parisi L, Pierelli F, Pozzessere G. A simple method for estimating conduction velocity of the spinothalamic tract in healthy humans. Clin Neurophysiol. 2000; 111:1907–1915. PMID:
11068222.

51. Low PA. Testing the autonomic nervous system. Semin Neurol. 2003; 23:407–421. PMID:
15088262.

52. Floeter MK. Cutaneous silent periods. Muscle Nerve. 2003; 28:391–401. PMID:
14506711.

53. Kim BJ, Kim NH, Kim SG, Roh H, Park HR, Park MH, et al. Utility of the cutaneous silent period in patients with diabetes mellitus. J Neurol Sci. 2010; 293:1–5. PMID:
20417526.

54. Vroomen PC, de Krom MC, Knottnerus JA. Diagnostic value of history and physical examination in patients suspected of sciatica due to disc herniation: a systematic review. J Neurol. 1999; 246:899–906. PMID:
10552236.

55. Vroomen PC, de Krom MC, Knottnerus JA. Consistency of history taking and physical examination in patients with suspected lumbar nerve root involvement. Spine (Phila Pa 1976). 2000; 25:91–96. discussion 97. PMID:
10647166.

56. Coster S, de Bruijn SF, Tavy DL. Diagnostic value of history, physical examination and needle electromyography in diagnosing lumbosacral radiculopathy. Neurol. 2010; 257:332–337.

57. Suri P, Rainville J, Katz JN, Jouve C, Hartigan C, Limke J, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine (Phila Pa 1976). 2011; 36:63–73. PMID:
20543768.

58. van der Windt DA, Simons E, Riphagen II, Ammendolia C, Verhagen AP, Laslett M, et al. Physical examination for lumbar radiculopathy due to disc herniation in patients with low-back pain. Cochrane Database Syst Rev. 2010; CD007431. PMID:
20166095.

59. Tong HC, Haig AJ, Yamakawa KS, Miner JA. Specificity of needle electromyography for lumbar radiculopathy and plexopathy in 55- to 79-year-old asymptomatic subjects. Am J Phys Med Rehabil. 2006; 85:908–912. quiz 913-915, 934. PMID:
17079963.

60. Daube JR, Rubin DI. Needle electromyography. Muscle Nerve. 2009; 39:244–270. PMID:
19145648.

61. Kim BJ, Date ES, Derby R, Lee SH, Seo KS, Oh KJ, et al. Electromyographic technique for lumbar multifidus examination: comparison of previous techniques used to localize the multifidus. Arch Phys Med Rehabil. 2005; 86:1325–1329. PMID:
16003658.

62. Haig AJ, Moffroid M, Henry S, Haugh L, Pope M. A technique for needle localization in paraspinal muscles with cadaveric confirmation. Muscle Nerve. 1991; 14:521–526. PMID:
1852159.

63. Stein J, Baker E, Pine ZM. Medial paraspinal muscle electromyography: techniques of examination. Arch Phys Med Rehabil. 1993; 74:497–500. PMID:
8489359.

64. Nardin RA, Raynor EM, Rutkove SB. Fibrillations in lumbosacral paraspinal muscles of normal subjects. Muscle Nerve. 1998; 21:1347–1349. PMID:
9736070.

65. Date ES, Mar EY, Bugola MR, Teraoka JK. The prevalence of lumbar paraspinal spontaneous activity in asymptomatic subjects. Muscle Nerve. 1996; 19:350–354. PMID:
8606700.

66. Date ES, Kim BJ, Yoon JS, Park BK. Cervical paraspinal spontaneous activity in asymptomatic subjects. Muscle Nerve. 2006; 34:361–364. PMID:
16634054.

67. Dumitru D, Diaz CA, King JC. Prevalence of denervation in paraspinal and foot intrinsic musculature. Am J Phys Med Rehabil. 2001; 80:482–490. PMID:
11421515.

68. Dumitru D. Physiologic basis of potentials recorded in electromyography. Muscle Nerve. 2000; 23:1667–1685. PMID:
11054745.

69. Haig AJ. Paraspinal denervation and the spinal degenerative cascade. Spine J. 2002; 2:372–380. PMID:
14589468.

70. Date ES, Kim BJ. Kimura J, editor. Cervical and thoracic radiculopathies. Peripheral Nerve Diseases: Handbook of Clinical Neurophysiology. 2006. Vol 7:1st ed. New York: Elsevier;p. 601–612.
71. Dillingham TR, Pezzin LE, Lauder TD. Cervical paraspinal muscle abnormalities and symptom duration: a multivariate analysis. Muscle Nerve. 1998; 21:640–642. PMID:
9572244.

72. Pezzin LE, Dillingham TR, Lauder TD, Andary M, Kumar S, Stephens RR, et al. Cervical radiculopathies: relationship between symptom duration and spontaneous EMG activity. Muscle Nerve. 1999; 22:1412–1418. PMID:
10487908.

73. Dillingham TR, Pezzin LE, Lauder TD. Relationship between muscle abnormalities and symptom duration in lumbosacral radiculopathies. Am J Phys Med Rehabil. 1998; 77:103–107. PMID:
9558009.

74. Dillingham TR, Pezzin LE, Lauder TD, Andary M, Kumar S, Stephens RT, et al. Symptom duration and spontaneous activity in lumbosacral radiculopathy. Am J Phys Med Rehabil. 2000; 79:124–132. PMID:
10744185.

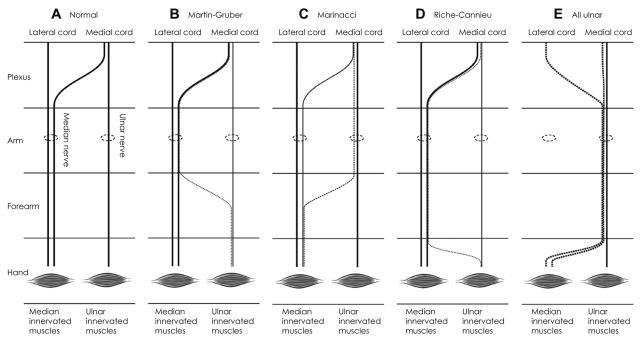
75. Srinivasan R, Rhodes J. The median-ulnar anastomosis (Martin-Gruber) in normal and congenitally abnormal fetuses. Arch Neurol. 1981; 38:418–419. PMID:
6454405.

76. Rodriguez-Niedenführ M, Vazquez T, Parkin I, Logan B, Sañudo JR. Martin-Gruber anastomosis revisited. Clin Anat. 2002; 15:129–134. PMID:
11877791.

77. Rodriguez-Niedenführ M, Vazquez T, Ferreira B, Parkin I, Nearn L, Sañudo JR. Intramuscular Martin-Gruber anastomosis. Clin Anat. 2002; 15:135–138. PMID:
11877792.

78. Lee KS, Oh CS, Chung IH, Sunwoo IN. An anatomic study of the Martin-Gruber anastomosis: electrodiagnostic implications. Muscle Nerve. 2005; 31:95–97. PMID:
15389650.

79. Leibovic SJ, Hastings H 2nd. Martin-Gruber revisited. J Hand Surg Am. 1992; 17:47–53. PMID:
1538112.

80. Amoiridis G, Vlachonikolis IG. Verification of the median-to-ulnar and ulnar-to-median nerve motor fiber anastomosis in the forearm: an electrophysiological study. Clin Neurophysiol. 2003; 114:94–98. PMID:
12495769.

81. Lee SA, Kim KK, Lee MC, Sunwoo IN, Kim KW. The electrodiagnostic findings in Martin-Gruber anastomosis. J Korean Neurol Assoc. 1994; 12:87–91.
82. Amoiridis G. Median--ulnar nerve communications and anomalous innervation of the intrinsic hand muscles: an electrophysiological study. Muscle Nerve. 1992; 15:576–579. PMID:
1584248.

83. Kimura J. Collision technique. Physiologic block of nerve impulses in studies of motor nerve conduction velocity. Neurology. 1976; 26:680–682. PMID:
945515.

84. Amoiridis G, Schöls L, Przuntek H, Wöhrle J. Collision technique in Martin-Gruber anastomosis. Muscle Nerve. 1998; 21:1354–1356. PMID:
9736072.

85. Amoiridis G. Fact and fallacy in electrophysiological studies of anomalous innervation patterns of the intrinsic hand muscles. Muscle Nerve. 1994; 17:245–247. PMID:
8114796.
86. Rubin DI, Dimberg EL. Martin-Gruber anastomosis and carpal tunnel syndrome: [corrected] morphologic clues to identification. Muscle Nerve. 2010; 42:457–458. PMID:
20665520.
87. Marras C, Midroni G. Proximal Martin-Gruber anastomosis mimicking ulnar neuropathy at the elbow. Muscle Nerve. 1999; 22:1132–1135. PMID:
10417799.

88. Whitaker CH, Felice KJ. Apparent conduction block in patients with ulnar neuropathy at the elbow and proximal Martin-Gruber anastomosis. Muscle Nerve. 2004; 30:808–811. PMID:
15316981.

89. Leis AA, Stetkarova I, Wells KJ. Martin-Gruber anastomosis with anomalous superficial radial innervation to ulnar dorsum of hand: a pitfall when common variants coexist. Muscle Nerve. 2010; 41:313–317. PMID:
19882647.

90. Simonetti S. Electrophysiological study of forearm sensory fiber crossover in Martin-Gruber anastomosis. Muscle Nerve. 2001; 24:380–386. PMID:
11353423.

91. Meenakshi-Sundaram S, Sundar B, Arunkumar MJ. Marinacci communication: an electrophysiological study. Clin Neurophysiol. 2003; 114:2334–2337. PMID:
14652092.

92. Harness D, Sekeles E. The double anastomotic innervation of thenar muscles. J Anat. 1971; 109(Pt 3):461–466. PMID:
5153804.
93. Boland RA, Krishnan AV, Kiernan MC. Riche-Cannieu anastomosis as an inherited trait. Clin Neurophysiol. 2007; 118:770–775. PMID:
17317302.

94. Kim BJ, Date ES, Lee SH, Lau EW, Park MK. Unilateral all ulnar hand including sensory without forearm communication. Am J Phys Med Rehabil. 2004; 83:569–573. PMID:
15213484.

95. Kudoh H, Sakai T, Horiguchi M. The consistent presence of the human accessory deep peroneal nerve. J Anat. 1999; 194(Pt 1):101–108. PMID:
10227671.

96. Lambert EH. The accessory deep peroneal nerve. A common variation in innervation of extensor digitorum brevis. Neurology. 1969; 19:1169–1176. PMID:
5389696.

97. Murad H, Neal P, Katirji B. Total innervation of the extensor digitorum brevis by the accessory deep peroneal nerve. Eur J Neurol. 1999; 6:371–373. PMID:
10210922.

98. Neundörfer B, Seiberth R. The accessory deep peroneal nerve. J Neurol. 1975; 209:125–129. PMID:
51049.
99. Owsiak S, Kostera-Pruszczyk A, Rowińska-Marcińska K. Accessory deep peroneal nerve - a clinically significant anomaly? Neurol Neurochir Pol. 2008; 42:112–115. PMID:
18512167.
100. Crutchfield CA, Gutmann L. Hereditary aspects of accessory deep peroneal nerve. J Neurol Neurosurg Psychiatry. 1973; 36:989–990. PMID:
4772729.

101. Sander HW, Quinto C, Chokroverty S. Accessory deep peroneal neuropathy: collision technique diagnosis. Muscle Nerve. 1998; 21:121–123. PMID:
9427233.

102. Kayal R, Katirji B. Atypical deep peroneal neuropathy in the setting of an accessory deep peroneal nerve. Muscle Nerve. 2009; 40:313–315. PMID:
19609929.

103. Linden D, Berlit P. The intrinsic foot muscles are purely innervated by the tibial nerve ("all tibial foot")--an unusual innervation anomaly. Muscle Nerve. 1994; 17:560–561. PMID:
8159190.
104. Lee SY, Yoon SR, Choi IS, Lee SG, Rowe SM. Extensor digitorum brevis innervated by the tibial nerve (all tibial foot): a case report. J Korean Acad Rehabil Med. 2000; 24:1223–1228.
105. Amoiridis G, Schöls L, Meves S, Przuntek H. Fact and fallacy in clinical and electrophysiological studies of anomalous innervation of the intrinsic foot muscles. Muscle Nerve. 1996; 19:1227–1229. PMID:
8761283.

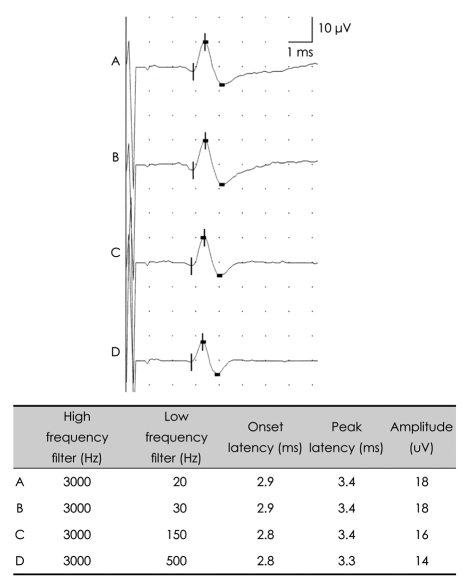
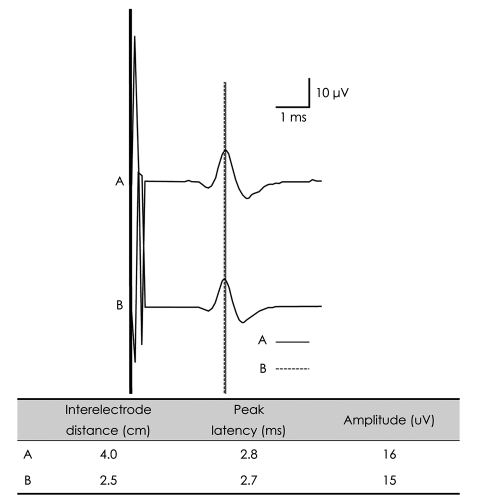

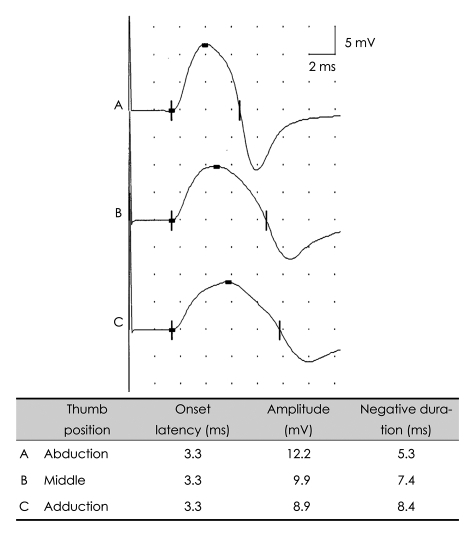
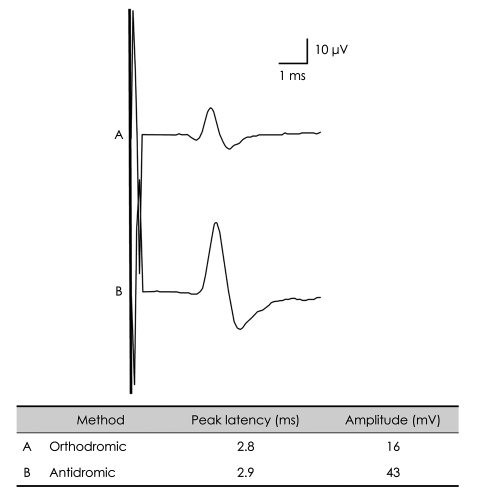




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download