Abstract
Background
There are conflicting findings regarding the association between hepatitis B (HB) virus (HBV) infection and atherosclerosis.
Case Report
A 34-year-old man was admitted for transient dysarthria and facial palsy. Ten years previously he had been diagnosed with HBV infection and treated with lamivudine (100 mg/day). Reactivation of HBV was detected 6 months before this recent admission. Serologic tests revealed that he was positive for HB early antigen, HB surface antigen, and anti-HB core. Brain magnetic resonance images were normal, but magnetic resonance angiograms revealed severe stenosis of the right middle cerebral artery, both external carotid arteries, and the basilar artery.
Hepatitis B (HB) virus (HBV) infection has generally been used to test the infection-atherosclerosis hypothesis due to several of its characteristics.1-3 For example, HBV can cause immune responses and may colonize the vascular tissues. In addition, it can cause thrombotic states, such as obliteration of the small portal and hepatic veins due to phlebitis and thrombosis associated with the disease progression.3,4 However, despite these characteristics, it has been recognized that the findings regarding the association between HBV infection and atherosclerosis are conflicting. Furthermore, because previous studies have usually been based on the general population, it is not yet known how atherosclerosis or ischemic stroke is related to HBV infection.5,6
We describe herein a case of multiple cerebral arterial stenoses associated with HBV infection, and discuss the role of HBV infection in the development of atherosclerosis.
A 34-year-old man was admitted to our tertiary hospital for transient dysarthria and facial weakness. He had experienced slurred speech, left facial weakness, and dizziness for several hours. His symptoms had completely recovered and he had no neurological abnormalities at admission. There were no vascular risk factors including hypertension, diabetes, hyperlipidemia, smoking, alcohol drinking, or a family history of vascular diseases. Ten years previously this patient had been diagnosed with HBV infection and treated with lamivudine (100 mg/day) at a local clinic. Six months before admission to our hospital he had suffered abdominal discomfort, nausea, and fatigue, at which time reactivation of HBV was detected. He was seropositive for HB early antigen (Ag) and HB surface (HBs) Ag, antibodies against these Ags, and HB core antibodies. Thereafter, the treatment was changed from lamivudine to entecavir (1 mg/day). Serologic tests performed in our hospital revealed that this patient remained positive for HB early Ag, HBs Ag, and HB core antibodies. Findings of laboratory tests including cholesterol profiles, C-reactive protein, D-dimer, fibrin degradation product, Venereal Disease Research Laboratory test, Helicobacter pylori Ag (enzyme-linked immunosorbent assay), and immunoglobulin M of Chlamydia pneumonia were normal. Screening tests for autoimmune and coagulation diseases including erythrocyte sedimentation rate, antinuclear antibody, anticardiolipin antibody, rheumatoid factor, lupus anticoagulant, anti-dsDNA, anti-Ro, anti-La, anti-neutrophilic cytoplasmic antibody, C3, C4, prothrombin time, activated partial thromboplastin time, protein-C, protein-S, and antithrombin-III also produced normal results. Brain magnetic resonance (MR) images were normal, but MR angiograms revealed severe stenosis of the right middle cerebral artery, both external carotid arteries, and the basilar artery. Conventional cerebral angiograms also demonstrated that the patient had eccentric and irregular stenoses in the same arteries (Fig. 1). Abdominal and aortic angiograms for differential diagnosis of vasculitis such as Takayasu's arteritis, fibromuscular dysplasia, and/or polyarteritis nodosa (PAN) yielded normal findings. Arterial stiffness was slightly higher compared to the norm for healthy men aged 34 years. The patient was treated with methylprednisolone (500 mg/day for 5 days, then tapered and stopped), entecavir (1 mg/day), and combined antiplatelet medication (aspirin and clopidogrel). Although transcranial Doppler performed 6 months later revealed that there was no improvement in flow velocity at the stenotic arteries, the patient had not developed any ischemic symptoms.
This case report reveals that multiple intracranial arterial stenoses were observed in association with HBV reactivation in a young patient without conventional vascular risk factors. The patterns of arterial stenosis and collateral supplies suggested that arterial stenoses develop due to long-standing atherosclerosis. There have been no reports about intracranial arterial stenosis and/or multiple cerebral arterial stenosis associated with HBV infection in young adults. One possible differential diagnosis in this case could be reversible cerebral vasoconstriction syndrome. This patient had been taking an antiviral agent when he developed neurological symptoms, which makes antiviral-drug-induced vasculitis a possible cause of this case. However, follow-up transcranial Doppler revealed that there was no improvement of flow velocity following appropriate treatment. Furthermore, atherosclerosis is usually shown to be eccentric and irregular stenosis.7 Our patient had eccentric and irregular stenosis of the middle cerebral artery and basilar artery, and so his atherosclerosis may have been caused by chronic and reactivated HBV infection.
We presumed that HBV had recently reactivated and played a role in the progression and destabilization of atherosclerotic plaques in multiple cerebral arteries. Several possible mechanisms could explain the relationship between HBV infection and atherosclerosis. First, because HBV is an intracellular organism, it may invade the vascular system.2,8 In order to confirm this hypothesis, pathologic studies that were not available for this case should be performed. Second, HBV infection has occasionally been associated with vasculitis. A substantial proportion of cases of PAN are related to HBV infection.9,10 However, in our case there was no evidence of either PAN or any other vasculitis. Finally, chronic HBV infection may stimulate inflammatory and immune-mediated responses.11 There was no laboratory evidence in our patient of an immune-mediated response, such as an elevated erythrocyte sedimentation rate. However, because reactivation of HBV infection started 6 months before admission to our hospital, this is a possible mechanism underlying the development of multiple arterial stenoses. The clinical course of chronic HBV infection is characterized by a series of exacerbations and remissions. Our patient with multiple arterial stenoses presented with transient dysarthria and facial palsy during the reactivation of HBV. Although no baseline MR images were acquired before the HBV reactivation, timely association of the HBV reactivation and the ischemic events led us consider a possible pathogenic link.
Previous studies have shown that certain infectious agents play an important role in atherogenesis.12,13 However, it is unclear whether atherogenesis is associated with any infection that provokes activation of inflammatory processes or whether only specific organisms play an important role. In addition, some investigators have reported that the activation of an inflammatory process, rather than specific infectious agents, might be responsible for the development of atherosclerotic diseases.14,15 Ishizaka et al.5 recently investigated the possible association between HBs Ag positivity and carotid arteriosclerosis. In contrast, Kiechl et al.14 found no significant association between chronic hepatitis and the presence of carotid atheromatous plaques, but it has also been shown that HBV infection has several features that make it potentially atherogenic.6 These conflicting results6,14 make large population studies necessary for clarification.
In conclusion, we describe herein a patient who developed multiple cerebral arterial stenoses associated with HBV infection. This case suggests that patients with HBV infection can develop multiple cerebral arterial stenoses even though they have no risk factors for this disease. However, because there are some conflicting findings, further studies are needed to confirm the association between HBV infection and atherosclerosis.
Figures and Tables
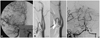 | Fig. 1Conventional cerebral angiogram. A: Right carotid angiogram showing severe focal stenosis in the midportion of the middle cerebral artery (arrows) that is eccentric and irregular. B: Carotid angiogram showing stenosis of both external carotid arteries (dotted arrows). C: Right vertebral arteriogram showing severe stenosis of the proximal basilar artery and left distal vertebral artery (arrows). |
References
1. Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation. 1999. 100:e20–e28.
2. Fattovich G, Brollo L, Giustina G, Noventa F, Pontisso P, Alberti A, et al. Natural history and prognostic factors for chronic hepatitis type B. Gut. 1991. 32:294–298.

3. Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995. 21:1238–1247.

4. Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993. 328:1828–1835.

5. Ishizaka N, Ishizaka Y, Takahashi E, Toda Ei E, Hashimoto H, Ohno M, et al. Increased prevalence of carotid atherosclerosis in hepatitis B virus carriers. Circulation. 2002. 105:1028–1030.

6. Sung J, Song YM, Choi YH, Ebrahim S, Davey Smith G. Hepatitis B virus seropositivity and the risk of stroke and myocardial infarction. Stroke. 2007. 38:1436–1441.

7. Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009. 72:627–634.

8. Mason A, Wick M, White H, Perrillo R. Hepatitis B virus replication in diverse cell types during chronic hepatitis B virus infection. Hepatology. 1993. 18:781–789.

9. Deeren DH, De Backer AI, Malbrain ML, Verbraeken H, Blockmans D. Treatment of hepatitis B virus-related polyarteritis nodosa: two case reports and a review of the literature. Clin Rheumatol. 2004. 23:172–176.

10. Trepo C, Guillevin L. Polyarteritis nodosa and extrahepatic manifestations of HBV infection: the case against autoimmune intervention in pathogenesis. J Autoimmun. 2001. 16:269–274.

11. Baig S, Alamgir M. The extrahepatic manifestations of hepatitis B virus. J Coll Physicians Surg Pak. 2008. 18:451–457.
12. Muhlestein JB. Chronic infection and coronary artery disease. Med Clin North Am. 2000. 84:123–148.

13. Saikku P, Leinonen M, Mattila K, Ekman MR, Nieminen MS, Mäkelä PH, et al. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988. 2:983–986.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download