Abstract
Background
Livedoid vasculitis is a chronic dermatological problem with an unclear etiology. Clinical findings are petechiae with painful ulcers in both lower extremities, which heal to become hyperpigmented and porcelain-white satellite lesions. There are only a few reported cases of livedoid vasculitis presenting in combination with peripheral neuropathy.
Livedoid vasculitis is a rare disease that has the clinically characteristic symptoms of purpuric macules and papules of the lower legs and feet. These purplish lesions develop into irregularly shaped ulcers that ultimately heal into stellate white scars or atrophie blanche. It is most common in young to middle-aged women, and the course seems to be chronic with seasonal exacerbations.1 Because of these characteristic findings it has many other synonyms, such as segmental hyalinizing vasculitis, atrophie blanche, livedo reticulitis with summer/winter ulceration, hypersensitivity-type vasculitis, and painful purpuric ulcers with reticular pattern of the lower extremities.2 Although the typical dermatological findings and pathogenesis of this condition have been emphasized previously, the involvement of peripheral neuropathy with this dermatological vasculopathy is very rare.3-5
A 48-year-old woman presented with a 2-year history of tingling sensation and numbness of her right fingers. Two years prior to her hospital visit, she began to notice that her right ring finger and little finger were numb and tingling; 7 months later, bruises suddenly appeared on her whole body, and these persisted only on both legs. These multiple purpuric patches developed into painful ulcerated wounds located mostly on the left lateral malleolar areas. The ulcers healed within 2 months, leaving atrophic scars. More and more similar skin lesions developed on both lower extremities, exhibiting a waxing and waning course. During this time, the patient also complained of num-bness and a tingling sensation on the dorsum of both feet, which was more severe on the right.
The patient's family and social history were unremarkable. Her previous medical history included a diagnosis of multiple cysts in the kidney and pancreas. She had also undergone a distal pancreatectomy; the removed tissue had pathologically confirmed serous oligocystic adenoma. There was no drug history, including use of an oral contraceptive pill.
When initially evaluated in our hospital, her cranial nerve function, motor function including muscle bulk, tone, power and deep-tendon reflex, cerebellar function test, and gait were all normal. However, a sensory examination revealed about a 50% hypesthesia for touch and pinprick sensation on the dorsum of both feet, the right fourth and fifth fingers, and the medial palm, which is innervated by the ulnar nerve. Multiple irregularly shaped ulcerative skin lesions and eschar with some healed ivory-white colored atrophic scars were seen in the left lateral malleolar area (Fig. 1). The results of the following laboratory studies regarding autoimmune disease and coagulation defects were normal, negative, or nonspecific: complete blood count, routine blood chemistry with blood glucose, urinalysis, serological test for syphilis, human immunodeficiency virus, and hepatitis B, sedimentation rate, antinuclear antibody, anti-double-stranded DNA antibodies, antineutrophilic cytoplasmic antibodies, antiphospholipid antibody, anticardiolipin antibody, anti-Sjögren's syndrome (SS)A/SSB antibody, protein C, protein S, cryoglobulin, rheumatoid factor, thyroid function test, vitamin B12, folate, creatine kinase, platelet aggregation panel, antithrombin III activity, gene study of factor V Leiden mutation, and prothrombin 20210 G>A. A cerebrospinal-fluid examination and Doppler ultrasound of both lower extremities produced normal results. The initial nerve conduction study (Table 1) revealed a diminished right ulnar sensory nerve action potential, and both sural sensory nerve action potentials were absent, even with repetitive stimulation. The amplitudes of the compound motor nerve action potentials of both the peroneal and posterior tibial nerves were decreased. Other motor and sensory conduction results were normal. A electrophysiological study revealed no abnormal temporal dispersion or partial conduction block.
Skin-punch biopsy sampling was conducted on the left lateral ankle. The findings were consistent with livedoid vasculopathy with fibrin deposition within the vessel walls, and thrombi and scattered perivascular lymphocytic inflammation.
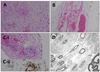
A nerve biopsy sample was removed from the right sural nerve, which revealed endoneurial capillary ectasia and congestion with hemorrhage, with extensive infarct of the peripheral nerve and Schwann cells. Occasional perivascular lymphocytes were observed infiltrating the arterioles in the epineurium. A marked degeneration of axons and the myelin sheath were observed, and the Schwann cell cytoplasm contained many autophagic vacuoles and myelin figures (Fig. 2). There was no definite evidence of vasculitis in the form of vessel necrosis and leukocytoclasia. The biopsy findings were suggestive of ischemic peripheral neuropathy.
This patient was diagnosed with ischemic neuropathy associated with livedoid vasculitis and treated with the antithrombotic and antifibrinolytic agents pentoxifylline and clopidogrel, respectively. The patient suffered no further attacks during the subsequent 3-year follow-up period.
While the name livedoid vasculitis is generally used, histopathological studies have shown that this condition can be more accurately described as livedoid vasculopathy. Typical histopathological findings of livedoid vasculitis are fibrin deposition within the affected walls of blood vessels and thrombus formation within the lumen. The extent of any inflammation is very limited.6,7 These findings were consistent with our patient's skin and nerve biopsy findings, showing ischemic neuropathy more than vasculitic neuropathy.
Livedoid vasculitis has been described as a sole entity or in association with other diseases, such as primary systemic lupus erythematosus, antiphospholipid antibody syndrome,8,9 and other conditions associated with abnormalities of the coagulation system (e.g., protein C deficiency,10 abnormalities of the tissue plasminogen activator system,11 antithrombin III deficiency,12 elevated homocysteine levels,13 and factor V Leiden mutations).14 Although the pathogenesis of livedoid vasculitis between vaso-occlusive and vasculitis continues to be debated, these pathologic and etiological studies lean toward the vaso-occlusive mechanism as a primary pathogenesis.15
The sequential nature of the numbness and tingling sensation in the patient's right hand and both feet is a classic feature of mononeuropathy multiplex. This is supported by her electrophysiological findings. Many systemic diseases can be related to mononeuropathy multiplex, such as vasculitis, connective tissue disease, cryobulinemia, sarcoidosis, diabetes, amyloidosis, neoplasm, and infections. The patient exhibited mononeuropathy multiplex with characteristic skin lesions, and so we could limit the disease entity to connective tissue diseases such as systemic lupus erythematosus, SS, and antiphospholipid antibody syndrome, and vasculitis conditions such as polyarteritis nodosa, infectious diseases such as leprosy or syphilis, and livedo vasculopathy. However, our laboratory results did not support the presence of any connective tissue diseases or vasculitis. Furthermore, the skin lesions that formed petechiae - which comprised recurrent, irregularly shaped, hyperpigmented ulcers and atrophie blanche - also differed from the derma-tological findings of both leprosy and syphilis. Leprosy is usually associated with more hypopigmented and well-defined lesions, with occasional plaque and tuberculoid-like changes.16 Syphilis characteristically presents hard, slightly elevated, round ulcers in the mouth and genital area known as chancres, and the subsequent formation of gummata.17 With the aid of an exclusive diagnostic process and examination of histopathological findings, we concluded that the patient was suffering from livedoid vasculopathy, and more specifically, livedoid vasculitis. Her mononeuropathy multiplex can be explained by multifocal axonal damage from thrombotic obstruction of the vasa nervorum and the consequent ischemia.
There is no consensus for the treatment of livedoid vasculitis. However, given its potential pathogenesis of coagulation abnormality and ischemia phenomenon, many antithrombotic and antifibrinolytic agents have been routinely used, including heparin,18 tissue plasminogen activator,19 pentoxifylline, aspirin, and dipyridamole,20 with long-term anticoagulation,21 danazol,22 ketanserin,23 beraprost,12 and prostacyclin.24 Other immunomodulating therapies such as steroids and immunoglobulins have also been applied, but it has been shown that they do not provide long-term benefits.25
We report herein a patient who experienced livedoid vasculitis combined with a rare neurological disease, mononeuropathy multiplex, which was proven to be ischemic neuropathy by nerve biopsy. These phenomena are thought to be attributable to a coagulation abnormality, which is also the main pathological cause in livedoid vasculitis. Other causes of coagulation defects were not revealed in this case. To the best of our knowledge, this is one of only very few reports of ischemic neuropathy associated with livedoid vasculitis, and has provided valuable insights into the disease pathomechanism. Our pathologic findings and other laboratory findings were all negative for systemic vasculitis, thus supporting the vaso-occlusive mechanism of livedoid vasculitis.
Figures and Tables
Fig. 1
Multiple painful ulcerations with healed white scarring in the bilateral lateral malleolar area. A: left lateral malleolar area. B: right lateral malleolar area.
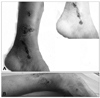
Fig. 2
Histopathology findings of a sural nerve biopsy. A: Extensive infarct of the peripheral nerve and Schwann cells (hematoxylin-eosin stain, original magnification ×40). B: Endoneurial capillary ectasia and congestion with hemorrhage (hematoxylin-eosin stain, original magnification ×200). C: Intravascular thrombosis with mild lymphocytic infiltration present in the epineurium. There was no definite neutrophilia or leukocytoclasia, suggesting vasculitis (i: hematoxylin-eosin stain, ii: leukocyte common antigen stain, original magnification ×200). D: Electron micrograph showing marked degeneration of axons and their myelin sheath, with the Schwann cell cytoplasm containing many autophagic vacuoles and myelin figures.
Acknowledgements
This work was supported by Grant No.0420100210 from the Seoul National University Hospital Research Fund.
References
1. Bard JW, Winkelmann RK. Livedo vasculitis. Segmental hyalinizing vasculitis of the dermis. Arch Dermatol. 1967. 96:489–499.

2. Papi M, Diodona B, De Pità O, Silvestri L, Ferranti G, Gantcheva M, et al. PURPLE (atrophie blanche): clinical, histological and immunological study of twelve patients. J Eur Acad Dermatol Venereol. 1997. 9:129–133.

3. Winkelmann RK, Schroeter AL, Kierland RR, Ryan TM. Clinical studies of livedoid vasculitis: (segmental hyalinizing vasculitis). Mayo Clin Proc. 1974. 49:746–750.
4. Toth C, Trotter M, Clark A, Zochodne D. Mononeuropathy multiplex in association with livedoid vasculitis. Muscle Nerve. 2003. 28:634–639.

5. Osada S, Kimura Y, Kawana S. Case of livedoid vasculopathy with peripheral neuropathy successfully treated with low-dose warfarin. J Dermatol. 2010. 37:98–101.

7. Milstone LM, Braverman IM, Lucky P, Fleckman P. Classification and therapy of atrophie blanche. Arch Dermatol. 1983. 119:963–969.

8. Grattan CE, Burton JL, Boon AP. Sneddon's syndrome (livedo reticularis and cerebral thrombosis) with livedo vasculitis and anticardiolipin antibodies. Br J Dermatol. 1989. 120:441–447.

9. Grob JJ, Bonerandi JJ. Thrombotic skin disease as a marker of the anticardiolipin syndrome. Livedo vasculitis and distal gangrene associated with abnormal serum antiphospholipid activity. J Am Acad Dermatol. 1989. 20:1063–1069.
10. Baccard M, Vignon-Pennamen MD, Janier M, Scrobohaci ML, Dubertret L. Livedo vasculitis with protein C system deficiency. Arch Dermatol. 1992. 128:1410–1411.

11. Pizzo SV, Murray JC, Gonias SL. Atrophie blanche. A disorder associated with defective release of tissue plasminogen activator. Arch Pathol Lab Med. 1986. 110:517–519.
12. Tsutsui K, Shirasaki F, Takata M, Takehara K. Successful treatment of livedo vasculitis with beraprost sodium: a possible mechanism of thrombomodulin upregulation. Dermatology. 1996. 192:120–124.

13. Gibson GE, Li H, Pittelkow MR. Homocysteinemia and livedoid vasculitis. J Am Acad Dermatol. 1999. 40:279–281.

14. Calamia KT, Balabanova M, Perniciaro C, Walsh JS. Livedo (livedoid) vasculitis and the factor V Leiden mutation: additional evidence for abnormal coagulation. J Am Acad Dermatol. 2002. 46:133–137.

15. Khenifer S, Thomas L, Balme B, Dalle S. Livedoid vasculopathy: thrombotic or inflammatory disease? Clin Exp Dermatol. 2010. 35:693–698.

17. Ooi C, Dayan L. Syphilis. Diagnosis and management in general practice. Aust Fam Physician. 2002. 31:629–635.
18. Jetton RL, Lazarus GS. Minidose heparin therapy for vasculitis of atrophie blanche. J Am Acad Dermatol. 1983. 8:23–26.

19. Klein KL, Pittelkow MR. Tissue plasminogen activator for treatment of livedoid vasculitis. Mayo Clin Proc. 1992. 67:923–933.

20. Drucker CR, Duncan WC. Antiplatelet therapy in atrophie blanche and livedo vasculitis. J Am Acad Dermatol. 1982. 7:359–363.

21. Dedichen J, Gjessing HC. Livedo reticularis with summer ulcerations; report of case treated with long-term anticoagulation therapy. Acta Med Scand Suppl. 1956. 319:74–78.
22. Hsiao GH, Chiu HC. Livedoid vasculitis. Response to low-dose danazol. Arch Dermatol. 1996. 132:749–751.
23. Rustin MH, Bunker CB, Dowd PM. Chronic leg ulceration with livedoid vasculitis, and response to oral ketanserin. Br J Dermatol. 1989. 120:101–105.

24. Hoogenberg K, Tupker RA, van Essen LH, Smit AJ, Kallenberg CG. Successful treatment of ulcerating livedo reticularis with infusions of prostacyclin. Br J Dermatol. 1992. 127:64–66.

25. Amital H, Levy Y, Shoenfeld Y. Use of intravenous immunoglobulin in livedo vasculitis. Clin Exp Rheumatol. 2000. 18:404–406.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download