Abstract
Background and Purpose
The occurrence of PWD in neurodegenerative disease is very rare, and this is the first report of it being related to early-onset AD. We describe a patient with early-onset Alzheimer's disease (AD) who presented with pure word deafness (PWD).
Pure word deafness (PWD) is a cortical auditory disorder that is characterized by defective comprehension of spoken language with a preserved ability to hear nonverbal sounds and communicate using written language. The most common etiology of PWD is stroke, with other causes being encephalitis, head trauma, and seizure.1,2
PWD is a very rare condition associated with neurodegenerative disorders. A few cases in which PWD was caused by frontotemporal dementia or primary progressive aphasia have been reported.3,4 However, to our knowledge there are no reports of patients with Alzheimer's disease (AD) who have presented with PWD. We describe herein a patient with early-onset AD who initially presented with PWD.
A 59-year-old, right-handed female with 12 years of school education visited our clinic because of difficulties comprehending spoken language over the previous 2 years. She had been a housekeeper and her past medical history was unremarkable except for well-controlled hypertension of 5 years duration.
The patient complained of a difficulty understanding spoken language that had developed insidiously 2 years previously and had subsequently progressed gradually. The patient described spoken conversation as sounding like a noise, and sometimes buzzing, which was worse in crowded public areas. The patient was unable to understand spoken questions when interviewed, but was able to comprehend and give correct answers by lip reading or responding to gestural cues. She was unable to understand what television actors were saying while watching Korean dramas, and preferred watching foreign movies with Korean subtitles. The patient had no problems understanding the context or plot of subtitled movies. In contrast to her defects in verbal comprehension, the patient perceived environmental noises such as door bells or ringing telephones, oriented towards their sources, and responded appropriately.
Despite the patient's deficiencies in verbal comprehension, her family noticed no impairment in her abilities to express her own thoughts, recall events, get directions, or use public transportation to travel around Busan where she had been living. She did not have problems with household activities such as cooking and cleaning, financial management, or participating in social meetings.
A few months after the onset of the first symptom, the patient visited the otolaryngology clinic of a general hospital and was prescribed a hearing aid, which did not improve her comprehension deficits. At 1 year postonset, the patient began to have difficulties with simple mathematics, such as making errors while paying a bill at a shop. Her comprehension of spoken language declined significantly, so that she could barely understand what people around her were saying, but she was still able to read and communicate in writing. However, the patient's husband reported that her writing style changed, becoming rough, and that she often committed errors in spelling. The patient was sometimes forgetful, but not to the extent that it affected her daily life. She began to have difficulty recalling the names of familiar neighbors, although she recognized them visually. Despite these problems, the patient's demeanor remained appropriate and her personal and social relationships remained intact.
Upon examination, the patient scored 17 out of 30 on the Mini-Mental State, Examination (-4 for calculation -3 for recall, and -6 for language). The results for the remainder of her neurological examination were unremakable, except for mild rigidity in her right upper extremity. We administered the Korean version of the Western Aphasia Battery to the patient. Her spontaneous speech output was fluent, but was characterized by phonemic paraphasia and intermittent repetition of syllablesyllable suggestive of mild neurogenic stuttering. She exhibited severe impairments in comprehension (2.1/10) and repetition (0/10), and mild impairments in visual confrontational naming (52/60) and word generation (7/20). In contrast, the patient was able to read aloud and understand written words and sentences without problem. She was also able to communicate in writing, although she did make some literal errors.
A subsequent audiological examination with pure-tone threshold audiometry revealed a mild bilateral sensorineural hearing loss (pure-tone thresholds of 30 dB in the left ear and 35 dB in the right ear within the range of 500-2,000 Hz). Her speech audiometry thresholds were 50 and 60 dB in the left and right ears (i.e., higher than her pure-tone thresholds), and her speech discrimination ability was 32% at 80 dB in the left ear and 20% at 85 dB in the right ear. The patient's brainstem auditory evoked potential responses were normal.
The patient was able to recognize and identify six out of eight nonverbal stimuli from a tape recording of animal sounds including the noises made by a cat, dog, horse, bee, cow, pig, fowl, and owl. When requested to listen to music and then point to the name of the song being played on a written list, she correctly matched the written song title to the presented song in four out of six trials.
All instructions for neuropsychological tests had to be given in written form. The patient performed below the normal range on the Korean version of Boston Naming Test, ideomotor praxis, Rey figure-copying test, calculation, finger naming, and semantic subtest of controlled oral association word recall test. In a Navon figure task, she recognized only small letters in all five items, suggestive of simultanagnosia. She exhibited difficulty naming famous people (14/40), but was able to describe the occupations of 17 whom she was unable to identify by name.
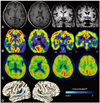
Routine laboratory tests revealed no evidence of comorbid disease. Brain MRI demonstrated mild atrophy in both temporal lobes, with the left side more affected. An 18FDG-PET scan revealed hypometabolism in bilateral temporoparietal areas, with greater effects on the left, suggestive of AD (Fig. 1A, B). To confirm the diagnosis of AD, we performed [11C] PIB-PET after obtaining informed consent, which revealed elevated PIB binding in diffuse cortical areas, especially in the temporoparieto-occipital cortices (Fig. 1C). We also compared the patient's cortical thickness to those of 100 healthy controls using surface-based morphometry with 3D volume MRI, as described previously.5 ANCOVA with an adjustment for age showed cortical thinning distributed within the bilateral Heschl's gyri, planum temporale, and superior temporal sulcus (Fig. 1D).
Our patient presented with verbal comprehension deficits combined with preservation of ability to hear, speak, read, write, and discriminate nonverbal environmental sounds; all of these findings are consistent with a diagnosis of PWD. The patient's comprehension deficits developed insidiously and progressed gradually over a period of 2 years. In addition to PWD, which is associated with bilateral temporal lesions, neuropsychological tests revealed apraxia, visuoconstructional disability, dysgraphia, impaired finger naming, and simultanagnosia, which are also suggestive of bilateral parietal lesions. All of the patient's symptoms and the clinical course of her disease were consistent with a diagnosis of neurodegenerative disease involving the bilateral temporoparietal areas.
Regarding differential diagnoses in this patient, we first considered the possibility of frontotemporal dementia. However, the patient did not show any abnormal behaviors characteristic of frontal lobe involvement such as disinhibition, abulia, or frontal-executive dysfunction on neuropsychological evaluation. The patient's low Korean version of Boston Naming Test score suggested early semantic dementia, but this was not consistent with the presence of parietal signs, the patient's intact ability to recognize people's faces on neuropsychological tests, and imaging findings. We also considered corticobasal degeneration, given the patient's prominent parietal dysfunctions, but excluded this possibility due to lack of asymmetric rigidity-akinesia or limb apraxia. Another possibility was a rare form of primary progressive aphasia.6 Although our patient began to show mild memory decline and errors in calculation in the earlier stage of the disease, she did not exhibit any functional difficulties in her daily living or social life, despite prominent language deficits in the first 2 years. In fact, PWD presented in only one of the six cases of slowly progressive aphasia without generalized dementia described in the original paper by Mesulam.6 Finally, we considered a diagnosis of early-onset AD (actually, a predementia stage of early-onset AD) given that her ability to participate in normal daily activities was largely intact. Moreover, increased PIB binding in diffuse cortical areas, particularly in temporoparieto-occipital areas, strongly supported the diagnosis of AD.
Regarding the lesion localization characteristic of PWD, the dominant or bilateral temporal lobes seem to be involved, although the precise neural correlates of PWD are not known.7,8 Coslett and colleagues suggested that PWD is associated with lesions in the bilateral auditory cortex, whereas perceptual defects of nonverbal auditory sound are related to other parts of the auditory system, such as the auditory association cortex.1 A previously described patient who presented with PWD after head trauma was found to have suffered a lesion in the planum temporale.9 Several cases of PWD caused by lesions located in areas other than the temporal cortex, including subcortical or midbrain lesions, have also been described.10,11 Furthermore, the planum temporale is related to phonetic analysis,12 and a lesion in the planum temporale is consistent with our patient's clincial characteristics. Our analysis of cortical thickness also supports this finding.
Two cases of PWD have been connected to focal atrophy syndromes including bilateral perisylvian and left superior temporal atrophy.3,4 The findings of our case suggest that PWD associated with AD is a manifestation of another focal atrophy syndrome. Our results also suggest that if a patient presents with PWD associated with a neurodegenerative etiology, the differential diagnoses should include frontotemporal dementia, AD, and primary progressive aphasia, where symptoms may be restricted to word deafness for the first several years with focal dominant atrophy in the left or bilateral superior temporal lobe and planum temporale.
Figures and Tables
Fig. 1
Brain MRI of the patient 2 years after the onset of pure word deafness. (A) Mild left temporal atrophy was suspected on axial and coronal scans. (B) 18FDG-PET demonstrated bilateral temporoparietal hypometabolism with greater involvement on the left side, and (C) [11C] PIB-PET showed increased PIB uptake in diffuse cortical areas. (D) A cortical thickness analysis showed cortical thinning in the bilateral Heschl's gyri, planum temporale, and superior temporal sulcus.
Acknowledgements
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare and Family Affairs, Republic of Korea (A050079).
References
1. Coslett HB, Brashear HR, Heilman KM. Pure word deafness after bilateral primary auditory cortex infarcts. Neurology. 1984. 34:347–352.

2. Denes G, Semenza C. Auditory modality-specific anomia: evidence from a case of pure word deafness. Cortex. 1975. 11:401–411.

3. Iizuka O, Suzuki K, Endo K, Fujii T, Mori E. Pure word deafness and pure anarthria in a patient with frontotemporal dementia. Eur J Neurol. 2007. 14:473–475.

4. Otsuki M, Soma Y, Sato M, Homma A, Tsuji S. Slowly progressive pure word deafness. Eur Neurol. 1998. 39:135–140.

5. Seo SW, Im K, Lee JM, Kim YH, Kim ST, Kim SY, et al. Cortical thickness in single- versus multiple-domain amnestic mild cognitive impairment. Neuroimage. 2007. 36:289–297.

6. Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982. 11:592–598.

7. Kaga K, Shindo M, Tanaka Y. Central auditory information processing in patients with bilateral auditory cortex lesions. Acta Otolaryngol Suppl. 1997. 532:77–82.

8. Kaga K, Shindo M, Tanaka Y, Haebara H. Neuropathology of auditory agnosia following bilateral temporal lobe lesions: a case study. Acta Otolaryngol. 2000. 120:259–262.

9. Wirkowski E, Echausse N, Overby C, Ortiz O, Radler L. I can hear you yet cannot comprehend: a case of pure word deafness. J Emerg Med. 2006. 30:53–55.

10. Hayashi K, Hayashi R. Pure word deafness due to left subcortical lesion: neurophysiological studies of two patients. Clin Neurophysiol. 2007. 118:863–868.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download