Abstract
Vestibular rehabilitation therapy (VRT) is an exercise-based treatment program designed to promote vestibular adaptation and substitution. The goals of VRT are 1) to enhance gaze stability, 2) to enhance postural stability, 3) to improve vertigo, and 4) to improve activities of daily living. VRT facilitates vestibular recovery mechanisms: vestibular adaptation, substitution by the other eye-movement systems, substitution by vision, somatosensory cues, other postural strategies, and habituation. The key exercises for VRT are head-eye movements with various body postures and activities, and maintaining balance with a reduced support base with various orientations of the head and trunk, while performing various upper-extremity tasks, repeating the movements provoking vertigo, and exposing patients gradually to various sensory and motor environments. VRT is indicated for any stable but poorly compensated vestibular lesion, regardless of the patient's age, the cause, and symptom duration and intensity. Vestibular suppressants, visual and somatosensory deprivation, immobilization, old age, concurrent central lesions, and long recovery from symptoms, but there is no difference in the final outcome. As long as exercises are performed several times every day, even brief periods of exercise are sufficient to facilitate vestibular recovery. Here the authors review the mechanisms and the key exercises for each of the VRT goals.
Most peripheral vestibular lesions have a benign etiology and undergo spontaneous resolution due to the self-limiting nature of the condition and the process of central nervous system (CNS) compensation.1 Vestibular compensation results from active neuronal changes in the cerebellum and brainstem in response to sensory conflicts produced by vestibular pathology.2 Occasionally, even in the absence of an ongoing vestibular lesion, poor compensation or maladaptive postural control strategies are adopted.2 Vestibular rehabilitation programs capitalize on the innate plasticity of the balance system to advance the natural compensation process.1
The earliest vestibular rehabilitation therapy (VRT), called the Cawthorne-Cooksey exercises, was developed by Cawthorne and Cooksey to treat patients with labyrinth injury resulting from surgery or head injury.3,4 They found that exercises designed to encourage head and eye movements hastened the patient's recovery. Their value in managing all forms of peripheral vestibular disorders rapidly became apparent, and they now form the mainstay of treatment for this group of patients. The exercises for vestibular rehabilitation can be categorized into two types: 1) physical therapy for vestibular hypofunction and 2) canalith repositioning therapy for benign paroxysmal positional vertigo (BPPV). This paper focuses on physical therapy for vestibular hypofunction, also known as VRT, balance rehabilitation therapy, and balance retraining therapy.
VRT is indicated for any condition characterized by a stable vestibular deficit, in which evaluation reveals no evidence of a progressive process and the patient's natural compensation process appears to be incomplete.2
Patients with stable CNS lesions or mixed central and peripheral lesions should not be excluded from treatment, although their prognoses are likely to be more limited than the average patient with a stable peripheral injury.2 A trend for the overall performance to be worse for mixed central-peripheral disease than for pure unilateral peripheral disease was seen, but no significant differences have been identified.
Patients with head injuries suffer from significant disability due to vestibular symptoms. Their conditions often include cognitive and central vestibular involvement along with a peripheral component. VRT techniques are therefore used as a supplement to a comprehensive, multidisciplinary head-injury program.2
Patients with panic disorder and other anxiety disorders often seek treatment for ill-defined vestibular symptoms. After appropriate evaluation is performed, VRT may be recommended as an adjunctive measure for their condition. If the anxiety is mild, VRT functions as a behavioral intervention similar to exposure therapy for the treatment of phobias. If the anxiety component is significant, and particularly if panic attacks are frequent, psychiatric intervention will also be required.2
In older adults with symptoms of dizziness and no documented vestibular deficits, the addition of vestibular-specific gaze stability exercises to standard balance rehabilitation results in a greater reduction in fall risk.5
It is not always possible for the physician to determine whether the patient's complaints are due to stable vestibular disease with inadequate central compensation or to unstable labyrinthine function.2 For the patients in whom the cause of vertigo is not clarified despite extensive diagnostic efforts, an empirical trial of vestibular physical therapy may be a helpful option. Identifying patients for whom the symptoms are not the direct result of a vestibular lesion does not prevent the use of vestibular rehabilitation as an adjunct treatment.6
Habituation will be almost impossible for an unstable lesion, and VRT is generally useless if the patient has ongoing labyrinthine pathology.2 Patients whose symptoms occur only in spontaneous episodes, such as seen with Ménière's disease, are unlikely to benefit from VRT. VRT is unsuccessful in patients with only spontaneously occurring events of disequilibrium, especially if the spontaneous vertigo or disequilibrium develops more than once per month.9 The primary objective in such patients is to prepare them for anticipated dizziness rather than to make any permanent change in their vestibular condition.10 Patients suspected of perilymphatic fistula whose condition deteriorates during exercise therapy are more likely to benefit from other treatments such as surgery.9
The symptoms and signs of acute vestibular neuritis are derived from static imbalance and dynamic disturbances in inputs from the semicircular canals and otolithic organs. Static imbalance refers to differences in the level of tonic discharge within the vestibular nuclei when the head is motionless, and dynamic disturbances refer to impaired compensatory responses during head movements.11 Static signs comprise nystagmus (semicircular canal origin), subjective visual vertical, subjective visual horizontal, ocular tilt reaction, and lateropulsion (otolithic sign). Dynamic signs include vestibulo-ocular reflex (VOR) asymmetry (semicircular canal sign), ocular counter-rolling, and postural instability (otolithic sign).12
Otolith dysfunction appears to improve more rapidly than canal-related impairments during short-term follow-up.13 Static signs and symptoms gradually abate within weeks, even in the presence of continued peripheral dysfunction. However, if vestibular function does not recover, dynamic signs will persist for life, leading to blurred vision and imbalance when patients turn their head toward the side of the affected labyrinth.14 Most patients will be able to walk within 48 hours, and most can return to normal activities within about 2 weeks. After 3 months, most will be as well as they are ever going to be, which is subjectively back to normal. At that time most patients will show only minor abnormalities of static vestibular function, such as 1-2 deg/s spontaneous nystagmus in darkness only, often accentuated by head shaking or application of vibration to the mastoid, a slight ipsilesional deviation of the subjective visual horizontal or subjective visual vertical, and rotation toward the side of the lesion during the stepping test.12 In general, improved function can be expected within 6 weeks, but time needed for function to improve increases with the duration of the problem.15 For patients who have undergone resection of an acoustic neuroma, their performance during the Romberg test with the eyes closed 3 days after surgery is predictive of whether they will benefit from brief vestibular adaptation exercises. This may be applied to patients with acute unilateral vestibular dysfunction.16 The recurrence rate of vestibular neuritis is low, and no recurrences are observed in the initially affected ear. However, a relapse may go undetected in those with a persistent and complete unilateral vestibular deficit.14 The symptoms may either worsen or be relieved during vestibular adaptation exercises; this seems to be a common pattern during improvement and is related to overactivity during the good days, which causes excessive fatigue resulting in increased symptoms within 24-36 hours.1 Even after vestibular compensation is achieved and symptoms are largely resolved, there may be occasional periods of symptomatic relapse due to decompensation. This may be triggered by a period of inactivity, extreme fatigue, a change in medications, or an intercurrent illness. A relapse of vestibular symptoms in this setting does not necessarily imply ongoing or progressive labyrinthine dysfunction.2
The overall mechanisms of recovery from vestibular lesions are vestibular adaptation and vestibular substitution. The vestibular adaptation approach is similar to that described by Cawthorne for patients with persistent disequilibrium.17 Vestibular adaptation involves readjusting the gain of the VOR or vestibulospinal reflex, whereas vestibular substitution employs alternative strategies to replace the lost vestibular function.12,18 The term "vestibular compensation" is used mostly as a synonym for vestibular substitution,17 but it is sometimes also used to describe the general recovery from unilateral vestibular deafferentiation syndrome. Thus, the term "well compensated" is used to describe fully functional recovery, while "poorly compensated" is applied to describe a partial recovery. The term "decompensation" is adopted to describe a near total relapse.19 A patient who describes a severe vestibular crisis at onset, with continuous disequilibrium or motion-provoked vertigo persisting or recurring, is probably uncompensated. This is true even though specific abnormalities are not apparent during vestibular testing.2
The goals of VRT are 1) enhancing gaze stability, 2) enhancing postural stability, 3) improving vertigo, and 4) improving daily living activities.20 The principles of VRT based on the goals thereof are described below.
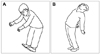
Gaze instability is due to the decreased gain of the vestibular response to head movements.20 The best stimulus for increasing the gain of the vestibular response is the error signal induced by retinal slip,18 which is the image motion on the retina during head motion.21 Retinal slip can be induced by horizontal or vertical head movements while maintaining visual fixation on a target. The target can be placed either within an arm's length or across the room (Fig. 1A).16 Repeated periods of retinal slip induce vestibular adaptation. However, not all head movements result in a VOR gain change. Horizontal (yaw plane) and vertical (pitch plane) head movements are effective, whereas head movements in the roll plane do not induce sufficient changes in the VOR gain.18
There are several ways to increase the effectiveness of vestibular adaptation during head movements. First, various amplitudes of retinal slip should be applied. Training that involves progressively increasing retinal slip errors is more effective than the use of sudden, large errors.22 To increase the magnification factor and the duration of exposure to retinal slip, the patient should view a target that is moving in the opposite direction of the head while moving the head either horizontally or vertically.18 Second, a wide range of head movement frequencies should be applied, because the greatest changes in VOR gain occur at the training frequencies.20,23 However, the training frequencies should not be changed abruptly. Adaptive changes in the VOR gain to retinal slip are greater when the error signal is gradually incremented than when it is only applied at its maximal level.24 Third, various directions of head movement should be employed, because this should provide an otolithic input that will influence the training effects.20,25 Patients should perform exercises for gaze stability four to five times daily for a total of 20-40 minutes/day, in addition to 20 minutes of balance and gait exercises.26 During the exercises to induce retinal slip, good visual inputs-such as bright room lights or with the curtains open-should be encouraged.27 There are also other ways to induce retinal slip, such as position error signals, imagined motion of the target, strobe lighting, and tracking of images stabilized on the retina (flashed after-images).22
While retinal slip is probably the most effective means of stimulating VOR adaptation, other error signals may also be used.22 Optokinetic visual stimuli also induce retinal slip,18 because smooth-pursuit eye movement itself is a part of the error signal.28 The benefit is that the optokinetic visual stimulus does not require head movements, and can be driven by oscillation of an optokinetic drum or of a light-emitting-diode stimulus.18 Unidirectional optokinetic training enhances vestibular responses in the corresponding direction. Thus, optokinetic or combined vestibular-optokinetic training may improve the VOR gain in unilateral peripheral vestibular dysfunction.29 During optokinetic visual stimuli, foveal and full-field stimuli work equally well in inducing adaptation.28
Even in the absence of a visual stimulus, the VOR gain can be raised to near-unity by asking the subject to imagine an earth-fixed target in darkness while moving the head. The VOR suppression can be trained by asking the subject to imagine a head-fixed target in darkness during head movements (Fig. 1B).30
Substitution by other eye-movement systems can effectively cancel the vestibular deficit and so protect the patient from perceiving smeared retinal images during head movements. Such substitution is possible when the patient has active control of the response.19 The other eye-movement systems are described below.
Corrective saccades become a part of the adaptive strategy to augment the diminished slow-phase component of the VOR.22 Two kinds of saccade may be found in patients with vestibular deficits. The first is a saccade of insufficient amplitude (undershoot). When the patient follows a target with the eyes and head, a saccade to the target of decreased amplitude (undershoot) is initially generated, and then the eyes drift to the target. This keeps the eyes in a fixed position during head rotation.31 The second type is a saccade back toward the target (pre-programmed saccade). During an ipsilesional unpredictable head rotation (yaw) away from a centrally positioned target, the saccade is generated in the opposite direction to the head rotation back toward the target.32
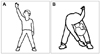
Smooth-pursuit eye movements can become a means of substitution for the deficient VOR. One study found that patients with a deficient vestibular system exhibited an enhancement in the pursuit system, with open- and closed-loop pursuit gains that were about 9% higher than those of the controls.33 Patients with severe bilateral vestibular loss also used smooth-pursuit eye movements to maintain gaze stability during head movements while fixating on a stationary target. Exercises for enhancing eye movements are shown in Fig. 2.18,34
Eye movements occur before the onset of the head rotation when the movement is anticipated. These eye movements are not vestibular in origin, but result from central preprogramming and efferent copy of the motor command.35 Visual acuity and VOR gains are better during predictable head movements toward the defect than those during unpredictable head movements. This infers that when the required movement is anticipated, central preprogramming is more effective for maintaining gaze stability.18 The use of central programming of eye movements to maintain gaze stability is greater among patients with bilateral vestibular loss than among healthy subjects or patients with unilateral vestibular loss.35
Both normal subjects and patients with unilateral vestibular deficits perform a blink during gaze saccades. This may prevent smear of the retinal image and cancel a VOR inadequacy.36
During low-frequency head movements (e.g., lower than 0.5 Hz), the cervico-ocular reflex (COR) caused the eye to rotate slowly in a direction opposite to the head movement.18 The COR makes no significant contribution to eye movements in normal subjects.37 However, in patients with bilateral vestibular loss, the COR takes on the role of the VOR in head-eye coordination by 1) initiating the anticompensatory saccade that takes the eyes in the direction of the target and 2) generating the subsequent slow compensatory eye movements.37 The COR has been known to contribute to gaze stability only in patients with bilateral vestibular loss, at least during low-frequency head movements (e.g., lower than 0.5 Hz).18 However, a recent study has revealed that the COR is also potentiated in patients with unilateral vestibular loss.38
Postural stability recovery is slower than gaze stability recovery.15 The primary mechanisms of postural recovery are increasing reliance on the visual and somatosensory cues (substitution) and improving the vestibular responses (adaptation).20 Recovery of normal postural strategies is required in patients with temporary deficits, while cases of permanent vestibular deficits need compensatory strategies, such as relying on alternative somatosensory cues.39 The goals of VRT, and especially for postural stability, are to help patients to 1) learn to use stable visual references and surface somatosensory information for their primary postural sensory system, 2) use the remaining vestibular function, 3) identify efficient and effective alternative postural movement strategies,40 and 4) recover normal postural strategies. For these, the therapist should assess whether the vestibular deficit is unilateral or bilateral, whether there is remaining vestibular function, whether the patient is overly reliant on particular sensory modalities such as vision or proprioception, and whether any other sensory impairment is present.41 The Clinical Test for Sensory Interaction in Balance was designed to assess how sensory information from the vestibular, visual, and somatosensory systems is used for postural stability.42 This test examines the patient's body sway while standing quietly for 20 seconds under the six different sensory conditions that alter the availability and accuracy of visual and somatosensory inputs for postural orientation.42 Somatosensory information is altered by having the patient stand on a slab of foam.42 Vision is eliminated with eye closure or blindfolds,42 or is altered by having the patient view the inside of dome (a modified Japanese lantern with vertical stripes inside) that is attached to the head.42 Nowadays, a moving visual surround is used instead of the dome to alter vision during the Clinical Test for Sensory Interaction in Balance.
Patients rely on somatosensory cues from the lower extremities during the acute stage, and on visual cues during the chronic stage.18 The visual inputs that arise from peripheral visual motion cues are more powerful than those from central (foveal) visual motion.40 Although visual cues become increasingly important, they can be very destabilizing as a postural reference in patients with vestibular loss. If visual cues to earth vertical are slowly moving or not aligned with gravity, the patient may align the body based on visual cues and thereby destabilize him- or herself, particularly when the surface reference is unstable or unavailable.40 This phenomenon is called visual dependency. When a patient is visually dependent, a moving visual scene (e.g., trucks passing in front of the patient in the street) can be misinterpreted as a self-motion, and the induced corrective postural adjustments can cause postural instability.41 Therefore, it is not optimal to foster visual dependency (e.g., by teaching the patient to fixate on a stationary object and to decrease head movements while walking).20
For patients who are visually dependent, exercises can be devised involving balancing with reduced or distorted visual input but good somatosensory inputs (e.g., in bare feet).41 These patients should practice maintaining balance during exposure to optokinetic stimuli such as moving curtains with stripes, moving discs with multicolored and differently sized circles, or even entire moving rooms.43 Exposure to optokinetic stimuli in the home environment may be accomplished by having the patient watch videos with conflicting visual scenes, such as high-speed car chases either on a video screen, busy screen savers on a computer, or moving large cardboard posters with vertical lines.43 Patients may watch a video showing visually conflicting stimuli while performing head and body movements and while sitting, standing, and walking.43
Somatosensory dependency may occur during vestibular recovery, especially in patients with bilateral vestibular deficits. In contrast to patients with unilateral vestibular deficit, patients with bilateral deficits rely on visual cues during the acute stage and somatosensory cues during the chronic stage.18,44 Vestibular compensation would not be expected to rely solely on visual inputs in such cases. In this situation the somatosensory cues are more important and could provide the requisite error signals leading to static rebalancing of the vestibular nuclei.11 This phenomenon is known as somatosensory dependency. To overcome this, patients should practice performing tasks while sitting or standing on surfaces with disrupted somatosensory cues for orientation, such as carpets, compliant foam, and moving surfaces (e.g., a tilt board). An example is catching a ball while standing on a carpet.43 Nevertheless, lost vestibular function cannot be fully substituted by visual and somatosensory cues.18
If a patient is unstable when both visual and somatosensory cues are altered, a treatment plan should be designed to improve the remaining vestibular function.45 Patients who are most confident in their balance ability and are better able to increase their vestibular weighting will be compensated the best.40 Thus, the ultimate goal for regaining postural stability is to help patients to learn to rely upon their remaining vestibular function as much as possible, and not to depend upon their vision and somatosensory function to substitute for the vestibular loss.40
It is necessary to gradually reduce or alter visual and somatosensory cues to teach patients to rely on their remaining vestibular function.15,41 Patients should practice maintaining a vertical position in the absence of visual or somatosensory cues with their eyes open and closed and on both firm and compliant surfaces. Patients need to practice walking in diverse environments, such as on grass, in malls, and during the night.15 Therefore, the exercises designed to improve postural balance are usually performed on a cushion with the eyes closed. The following exercises may also be included: 1) walking and turning suddenly or walking in a spiral path, and 2) walking while a therapist orders them to turn to the right or left.15 Exercises to improve balance in sitting and other positions are usually not necessary.15
Controlling the body position and orientation requires motor coordination processes that organize muscles throughout the body into coordinated movement strategies.43 These processes are postural strategies, and are described below.
Three main postural strategies are employed to recover balance during standing: ankle, hip, and step strategies. The ankle strategy involves standing in a wide stance and using ankle torques in a bottom-up, inverted-pendulum type of sway. The hip strategy involves standing in a narrow stance and using rapid torques around the trunk and hips in a top-down control.46 The ankle strategy is more dependent on somatosensory than vestibular function,46 while the hip strategy is more dependent on vestibular function.47 The ankle strategy involves moving the upper and lower parts of the body in the same direction or in phase, whereas the hip strategy requires that the upper and lower parts of the body move forward or backward in the opposite direction or out of phase. The step strategy is a stepping movement used when stability limits are exceeded.
Patients with vestibular loss use the ankle strategy but not the hip strategy, even when the hip strategy is required for postural stability, such as when standing on one foot, across a narrow beam, or in a heel-toe stance.46 Vestibular deficits may sometimes result in abnormally coordinated postural movement strategies that would give rise to excessive hip sway.48 This can cause a fall when the surface is slippery.41
Alternative postural strategies should be identified for patients using abnormally coordinated postural strategies. These patients should be retrained to use the redundancy within the balance system.41 Since the postural strategies are centrally programmed and can be combined according to postural conditions, subject expectations, and prior experiences,47 the patient should practice performing a given strategy during self-initiated sway, or during tasks involving voluntary limb movements or in response to perturbations.41
The ankle strategy can be practiced by swaying back and forth and side to side within small ranges, keeping the body straight and not bending at the hips or knees.43 Small perturbations are used, such as a small pull or push at the hips or shoulders. Patients perform various tasks, such as reaching, lifting, and throwing.43 The hip strategy may be practiced by maintaining balance without taking a step and making increasingly faster and larger displacements (Fig. 3). This can be facilitated by restricting the force control at the ankle joints by standing across a narrow beam, or standing heel to toe or in a single-limb stance. Patients can practice both voluntary sway and responses to external perturbations on altered surfaces.43 The step strategy can be practiced by the patient passively shifting his or her weight to one side and then quickly bringing the center of mass back towards the unweighted leg, or in response to large backward or forward perturbations.43 Patients can also practice stepping over a visual target or obstacle in response to external perturbations.43
The authors have experienced a patient with chronic vestibular loss who could ride a bicycle well despite having vertigo and imbalance while walking. This may be an example of the description by Brandt et al.49 of patients with acute vestibular disorders who are better at maintaining their balance when running than when walking slowly. This suggests that the automatic spinal locomotor program suppresses destabilizing vestibular inputs.49
Light touch that provides a somatosensory cue without mechanical support is a powerful sensory reference for postural control. Thus, the use of a cane, which acts as an extended haptic 'finger' for orientation to an earth reference, is an important tool for postural rehabilitation.40 Falling is an important consequence of bilateral vestibular hypofunction, and patients should be counseled about the increased risk of falling. Assistive devices should especially be considered for persons older than 65 years with bilateral vestibular loss.50 Unlike most patients with a unilateral disorder, those with bilateral vestibular deficits may require a walking aid, especially in the early stages. However, care should be taken to ensure that such patients do not become dependent on such aids.41
The authors experienced a blind man who did not wear caps or gloves even in cold weather, because when he did he experienced a feeling of losing balance. This suggests that somatosensory information from the face serves a compensation function.51 Therefore, it may be recommended that patients with balance disorders should avoid wearing caps or gloves when they are walking.
Patients with peripheral vestibular lesion employ compensatory strategies that involve decreasing their trunk and neck rotations in an effort to improve stability by avoiding head movements.52 Patients typically turn "en bloc", and may even stop moving before they turn. This can lead to secondary musculoskeletal impairments including muscle tension, fatigue, and pain in the cervical region, and sometimes also in the thoracolumbar region.52
Patients may not be able to actively achieve full cervical range of motion as a result of dizziness, pain, or cocontraction, although the passive range with the head supported against gravity might be maintained.52 Patients use excessive visual fixation and therefore have increased difficulty if asked to look up or turn their heads while walking.52 However, this strategy is not useful because it results in a limitation of normal activity and does not provide a mechanism for seeing clearly during head movements.18
Animal studies have produced evidence of spontaneous cellular recovery. Complete functional recovery of vestibular function was observed after streptomycin treatment in chicks, Gallus domesticus.53 Single-neuron studies also demonstrated that a significant recovery of resting activity occurs in the vestibular nuclei ipsilateral to the lesion by the time the spontaneous nystagmus and roll head tilt have largely disappeared.51 However, it is unclear whether this cellular recovery is a significant factor in the restoration of vestibular function in humans.18
If the peripheral lesion is extensive, the ipsilateral vestibular nucleus will become responsive to changes in the contralateral eighth-nerve firing rate by activating the commissural pathways.2 There may be adaptive substitution or compensation within the central vestibular system of the unaffected side. A beneficial result is the suppression of input from the affected modality and the restoration of adequate spatial orientation by the contralateral, unaffected vestibular nuclear complex.54 Corrective saccades occur at latencies that suggest that they could be triggered from neck proprioception or from changes in activity in the intact, contralateral vestibular afferents in the case of unilateral vestibular hypofunction.22
Improving the vertigo should be the primary goal in most patients with provoked positioning vertigo without a definite diagnosis but with a benign etiology.2 This can be achieved by habituation of abnormal vestibular responses to rapid movements.1 The therapist identifies the typical movements that produce the most intense symptoms and provides the patient with a list of exercises that reproduce these movements.2 The motion sensitivity test is used to assess the positions and movements that provoke symptoms. This test employs consecutive movements and positions such as turning the head or body during lying, sitting, or standing.15 Habituation is a reduction in the magnitude of the response to repetitive sensory stimulation, and it is induced by repetitive exposures to a provoking movement.43
Habituation is specific to the type, intensity, and direction of the eliciting stimuli. In most cases, the provoking movement is a less frequently executed movement during daily activities. Repetition of the originally abnormal signal will stimulate compensation.55 The therapist should sometimes distinguish pure BPPV from positional vertigo resulting from poor compensation after a labyrinthine injury.2 Provoked vertigo disappears when the central compensation stimulated by the exercise has developed sufficiently.43,56 After habituation, the spatial disorientation becomes the usual one and then begins to be integrated into the normal processing mechanism.55 If patients can persevere with their program, most will begin to notice dramatic relief of positional vertigo within 4-6 weeks.21 The habituation effect is slower for the aged and the end result may not be complete success in some patients.56 The habituation effect persists for a very long time after application of the stimulus.55 The Brandt-Daroff exercise is also a habituation therapy.57 The present authors have experienced many patients who experience vertigo induced by bending over their neck or trunk. The exercise for those patients is presented in Fig. 4.
Habituation exercises are inappropriate for patients with bilateral vestibular loss, because they are designed to decrease unwanted responses to vestibular signals rather than to improve gaze or postural stability.52 However, for those patients with bilateral vestibular deficits, the theoretical benefit to eye-head habituation activities (although not specifically tested) is a reduction in oscillopsia.1 Certain habituation exercises such as rising quickly should not be performed by the elderly, because they might induce orthostatic hypotension.15
The ultimate goal of vestibular recovery should be to enable the patient to return to all of his or her normal activities of daily living. Therefore, VRT is not considered to be complete until the patient has returned to normal work or is satisfactorily resettled. Patients who are unable to return to their normal work and in whom the disability is likely to last at least 6 months are considered to be disabled.4 To achieve the final goal of vestibular recovery, the exercise is integrated into normal activities such as walking,43 rather than being performed with the patient sitting or standing quietly.58 Various games can be introduced to reduce the monotony of purely remedial exercises.14 Patients who are gradually and safely exposed to a wide variety of sensory and motor environments are teaching their nervous systems to identify strategies to accomplish functional goals.40
All patients who receive customized VRT programs are also provided with suggestions for a general exercise program that is suited to their age, health, and interests. For most, this would at least involve a graduated walking program. For many, a more strenuous program is suggested that may include jogging, walking on a treadmill, doing aerobic exercises, or bicycling. Activities that involve coordinated eye, head, and body movements such as golf, bowling, handball, or racquet sports may be appropriate. Swimming should be approached cautiously because of the disorientation experienced by many vestibular patients in the relative weightlessness of the aquatic environment.2 Older adults who talk as they walk with assistive devices are more likely to fall than those who do not talk as they walk.59 Therefore, older patients should be instructed that when a conversation is started they should stop walking in order to prevent falling.59 If rapid head movements cause imbalance, the patients should be advised not to drive.60
Factors affecting recovery are medications, visual and somatosensory inputs, stage at which treatment is commenced, daily exercise duration, symptom intensity, the site of the lesion, the patient's age, and psychogenic factors.
The use of centrally acting medications such as vestibular suppressants, antidepressants, tranquilizers, and anticonvulsants has no adverse effect on the eventual therapy outcome. However, the mean duration of therapy required to achieve the eventual outcome is significantly longer in patients using medication.1,2,9
It was initially believed that the earlier patients commence exercises, the quicker and better the results.4,16,29 However, the lack of an adverse effect of duration of symptoms on therapy outcome argues against there being a critical period. It is currently accepted that there is no critical time period within which individuals achieve a significant functional improvement.9,26
Brief periods of unidirectional optokinetic stimulation (30 seconds, ten times daily for 10 days) can produce VOR gain changes after unilateral vestibular loss in humans.29 Therefore, we postulate that even brief periods of stimulation can induce recovery of vestibular function.
Symptom intensity does not influence the therapy outcome.1 However, if the lesion is unstable, such as a fluctuating vestibular deficit (e.g., Ménière's disease), incomplete damage, a positional phenomena (BPPV), ongoing labyrinthine pathology, or a slowly progressive tumor, it is difficult for the CNS to compensate, and exercise therapy is generally useless.9,39 Patients with spontaneous or continuous symptoms of disequilibrium, a history of head injury, permanent disability, or severe postural control abnormalities perform the poorest in existing therapy programs.9
Patients with a central or mixed lesion expect a prolonged period of therapy, but the final outcome does not vary with the location. Those with mixed lesion sites may require longer therapy,9,60 and patients with a pure central lesion demonstrated a trend for a more successful therapy outcome compared with those with mixed lesions.2 A lesion of the cerebellum delays recovery.61 Patients with a head injury and associated vestibular deficit show less improvement with treatment,62 and have a significantly worse outcome.1
Complicating features of anxiety, depression, or excessive dependence on medications may hinder vestibular compensation.2
Before commencing exercises, simple techniques for reducing neck tension may be needed (e.g., shoulder shrugging, shoulder/arm rotation, and gentle stretching exercises specific for the neck region).43 Head movements must be encouraged both to induce vestibular adaptation and to habituate the symptoms provoked by movement.15 Patients should practice a wide range of functional tasks in various contexts, including maintaining balance with a reduced base of support, maintaining balance while changing the orientation of the head and trunk, and maintaining balance while performing various upper-extremity tasks.43
The exercises for VRT include general strengthening and flexibility exercises, voluntary eye movements and fixations (visual stabilization exercises), active head movements (recalibration of the VOR), active body movements (improvement of vestibulospinal regulation), substitution exercises for the use of various senses (particularly somatosensory cues) and vision, visual-dependency exercises, somatosensory dependence exercises, habituation exercises, education for using assistive devices, and safety awareness techniques to avoid falls.64
Key exercises for enhancing gaze stability, enhancing eye movements, enhancing postural stability, and improving vertigo are described in Table 1.
The exercises can be modified by performing them under various conditions (Table 2).
Patients are typically seen once every 1-2 weeks and are provided with a specific daily home exercise program. During each visit the therapist addresses the specific problems and goals of the individual patient. As a patient's progress in therapy plateaus, he or she is switched from customized exercises to a maintenance program comprising a wide variety of motion-orientated activities.1
Once all of the exercises can be performed without dizziness, patients should maintain a high degree of physical activity (e.g., playing ball games or dancing) in order to sustain the achieved compensation.43 It should be kept in mind that after compensation is achieved, periods of stress, fatigue, or illness may result in a temporary recurrence of vertigo.43
A patient may experience nausea or vomiting in the first several hours after an acute vestibular lesion. This can be relieved by adequate medications. After the nausea or vomiting subsides, the patient quietly lies down and turns the head very slowly while looking at a target on the ceiling. If the patient can sit up, a few key exercises may be started.
When nausea or vomiting occurs during exercises, patients are advised to return to the performing the previous exercise on their programs until the nausea becomes prohibitive.1 At that point they should stop the program and begin again at the next scheduled time. When symptoms cannot be handled in this manner, antiemetic medication is used simultaneously. This approach is also used when the exercises stimulate prolonged periods of aggravated vertigo following the exercise activity, thereby disrupting daily routines. In this case, simultaneous administration of vestibular suppressants may be required.1
While good visual inputs are recommended, eye glasses can aggravate vertigo during head oscillation. The present authors recommend that in such cases eye glasses should not be worn during the exercise.
The environment must be modified to allow a patient to practice the exercises safely and without the continual supervision of a therapist.43 Therefore, patients who are very unsteady or fearful of falling should practice movements while wearing a harness connected to the ceiling, standing between parallel bars, standing close to a wall or corner, or standing with a chair or table in front of them.43
Exercises related to eye and head movements are key to improving gaze stability, whereas exercises performed while standing on a narrow base or a cushion with the eyes closed are key to improving postural stability. VRT is applicable to patients with stable vestibular lesions whose vestibular function is poorly compensated, regardless of their age, the cause of their lesion, and symptom duration and intensity. The use of centrally acting medications and visual/somatosensory deprivation should be avoided. Safety is a major concern, so that therapists should always monitor patients during a treatment session. Education and instruments for safety should always be accessible to patients. VRT reduces the cost of treating vertigo by reducing unnecessary medications and studies, and by shortening the recovery period. Indeed, VRT is safe, effective, and without reported adverse effects-it currently represents the most useful tool for the alleviation of protracted vertigo.
More information of specific exercise techniques and therapy program designs is available at http://retrainings.com.
Figures and Tables
 | Fig. 2Exercises for enhancing eye movements. A: Exercise for saccade and vestibulo-ocular reflex: 1, look directly at a target, ensuring that your head is aligned with the target; 2, look at the other target; and 3, turn your head to the other target. B: Exercise for imagery pursuit: 1, look directly at a target, ensuring that your head is aligned with the target; 2, close your eyes; 3, slowly turn your head away from the target while imagining that you are still looking directly at the target; and 4, open your eyes and check to see whether you have been able to keep your eyes on the target; if not, adjust your gaze on the target. |
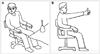
 | Fig. 3Swaying back and forth. A: Bend forward and move the center of your body backward with your toes up. B: Bend backward and move the center of your body forward with your heels up. Repeat several times. |
 | Fig. 4Exercises for improving vertigo. A: Stand with one arm elevated over the head, with the eyes looking at the elevated hand. B: Bend over and lower the arm diagonally with the eyes continuously looking at the hand until the hand arrives at the opposite foot. Repeat with the other arm. |
References
1. Shepard NT, Telian SA, Smith-Wheelock M. Habituation and balance retraining therapy. A retrospective review. Neurol Clin. 1990. 8:459–475.
2. Shepard NT, Telian SA. Programmatic vestibular rehabilitation. Otolaryngol Head Neck Surg. 1995. 112:173–182.
5. Hall CD, Heusel-Gillig L, Tusa RJ, Herdman SJ. Efficacy of gaze stability exercises in older adults with dizziness. J Neurol Phys Ther. 2010. 34:64–69.

6. Shepard N, Asher A. Herdman SJ, editor. Treatment of patients with nonvestibular dizziness and disequilibrium. Vestibular Rehabilitation. 2000. 2nd ed. Philadelphia: F.A. Davis Co.;534–544.
7. Seok JI, Lee HM, Yoo JH, Lee DK. Residual dizziness after successful repositioning treatment in patients with benign paroxysmal positional vertigo. J Clin Neurol. 2008. 4:107–110.

8. Blatt PJ, Georgakakis GA, Herdman SJ, Clendaniel RA, Tusa RJ. The effect of the canalith repositioning maneuver on resolving postural instability in patients with benign paroxysmal positional vertigo. Am J Otol. 2000. 21:356–363.

9. Shepard NT, Telian SA, Smith-Wheelock M, Raj A. Vestibular and balance rehabilitation therapy. Ann Otol Rhinol Laryngol. 1993. 102:198–205.

10. Hain TC. Vestibular rehabilitation therapy (VRT). cited 2010 Oct 3. Available from: http://www.dizziness-and-balance.com/treatment/rehab.html.
11. Zee DS. Herdman SJ, editor. Vestibular adaptation. Vestibular Rehabilitation. 2007. 3rd ed. Philadelphia: F.A. Davis Co.;77–90.
12. Halmagyi GM, Weber KP, Curthoys IS. Vestibular function after acute vestibular neuritis. Restor Neurol Neurosci. 2010. 28:37–46.

13. Kim HA, Hong JH, Lee H, Yi HA, Lee SR, Lee SY, et al. Otolith dysfunction in vestibular neuritis: recovery pattern and a predictor of symptom recovery. Neurology. 2008. 70:449–453.

14. Brandt T, Huppert T, Hüfner K, Zingler VC, Dieterich M, Strupp M. Long-term course and relapses of vestibular and balance disorders. Restor Neurol Neurosci. 2010. 28:69–82.

15. Herdman SJ, Whitney SL. Herdman SJ, editor. Intervention for the patient with vestibular hypofunction. Vestibular Rehabilitation. 2007. 3rd ed. Philadelphia: F.A. Davis Co.;309–337.
16. Herdman SJ, Clendaniel RA, Mattox DE, Holliday MJ, Niparko JK. Vestibular adaptation exercises and recovery: acute stage after acoustic neuroma resection. Otolaryngol Head Neck Surg. 1995. 113:77–87.

17. Krebs DE, Gill-Body KM, Riley PO, Parker SW. Double-blind, placebo-controlled trial of rehabilitation for bilateral vestibular hypofunction: preliminary report. Otolaryngol Head Neck Surg. 1993. 109:735–741.

18. Herdman SJ. Role of vestibular adaptation in vestibular rehabilitation. Otolaryngol Head Neck Surg. 1998. 119:49–54.

19. Curthoys IS, Halmagyi M. Herdman SJ, editor. Vestibular compensation: clinical changes in vestibular function with time after unilateral vestibular loss. Vestibular Rehabilitation. 2007. 3rd ed. Philadelphia: F.A. Davis Co.;172–194.
21. Gauthier GM, Robinson DA. Adaptation of the human vestibuloocular reflex to magnifying lenses. Brain Res. 1975. 92:331–335.

22. Schubert MC, Zee DS. Saccade and vestibular ocular motor adaptation. Restor Neurol Neurosci. 2010. 28:9–18.

23. Lisberger SG, Miles FA, Optican LM. Frequency-selective adaptation: evidence for channels in the vestibulo-ocular reflex? J Neurosci. 1983. 3:1234–1244.

24. Schubert MC, Della Santina CC, Shelhamer M. Incremental angular vestibulo-ocular reflex adaptation to active head rotation. Exp Brain Res. 2008. 191:435–446.

25. Tiliket C, Shelhamer M, Tan HS, Zee DS. Adaptation of the vestibulo-ocular reflex with the head in different orientations and positions relative to the axis of body rotation. J Vestib Res. 1993. 3:181–195.

26. Herdman SJ, Hall CD, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg. 2007. 133:383–389.

27. Fetter M, Zee DS, Proctor LR. Effect of lack of vision and of occipital lobectomy upon recovery from unilateral labyrinthectomy in rhesus monkey. J Neurophysiol. 1988. 59:394–407.

28. Shelhamer M, Tiliket C, Roberts D, Kramer PD, Zee DS. Short-term vestibulo-ocular reflex adaptation in humans. II. Error signals. Exp Brain Res. 1994. 100:328–336.
29. Pfaltz CR. Vestibular compensation Physiological and clinical aspects. Acta Otolaryngol. 1983. 95:402–406.
30. Barnes GR. Visual-vestibular interaction in the control of head and eye movement: the role of visual feedback and predictive mechanisms. Prog Neurobiol. 1993. 41:435–472.

31. Kasai T, Zee DS. Eye-head coordination in labyrinthine-defective human beings. Brain Res. 1978. 144:123–141.

32. Tian J, Crane BT, Demer JL. Vestibular catch-up saccades in labyrinthine deficiency. Exp Brain Res. 2000. 131:448–457.

33. Bockisch CJ, Straumann D, Hess K, Haslwanter T. Enhanced smooth pursuit eye movements in patients with bilateral vestibular deficits. Neuroreport. 2004. 15:2617–2620.

34. Leigh RJ, Huebner WP, Gordon JL. Supplementation of the human vestibulo-ocular reflex by visual fixation and smooth pursuit. J Vestib Res. 1994. 4:347–353.

35. Herdman SJ, Schubert MC, Tusa RJ. Role of central preprogramming in dynamic visual acuity with vestibular loss. Arch Otolaryngol Head Neck Surg. 2001. 127:1205–1210.

36. Black RA, Halmagyi GM, Curthoys IS, Thurtell MJ, Brizuela AE. Unilateral vestibular deafferentation produces no long-term effects on human active eye-head coordination. Exp Brain Res. 1998. 122:362–366.
37. Bronstein AM, Hood JD. The cervico-ocular reflex in normal subjects and patients with absent vestibular function. Brain Res. 1986. 373:399–408.

38. Schubert MC, Das V, Tusa RJ, Herdman SJ. Cervico-ocular reflex in normal subjects and patients with unilateral vestibular hypofunction. Otol Neurotol. 2004. 25:65–71.

39. Shumway-Cook A, Horak FB, Yardley L, Bronstein AM. Bronstein AM, Brandt T, Woollacott M, editors. Rehabilitation of balance disorders in the patient with vestibular pathology. Clinical Disorders of Balance Posture and Gait. 1996. London: Arnold;211–235.
40. Horak FB. Postural compensation for vestibular loss and implications for rehabilitation. Restor Neurol Neurosci. 2010. 28:57–68.

41. Shumway-Cook A, Horak FB. Luxon LM, Davies RA, editors. Physical exercise regimes-practical aspects. Handbook of Vestibular Rehabilitation. 1997. London: Whurr Publishers;101–115.
42. Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction of balance. Suggestion from the field. Phys Ther. 1986. 66:1548–1550.
43. Pavlou M, Shumway-Cook A, Horak FB, Yardley Yardley, Bronstein AM. Bronstein AM, Brandt T, Woollacott MH, Nutt JG, editors. Rehabilitation of balance disorders in the patient with vestibular pathology. Clinical Disorders of Balance, Posture and Gait. 2004. 2nd ed. London: Arnold;317–343.
44. Bles W, Vianney de Jong JM, de Wit G. Compensation for labyrinthine defects examined by use of a tilting room. Acta Otolaryngol. 1983. 95:576–579.

45. Whitney SL, Herdman SJ. Herdman SJ, editor. Physical therapy assessment of vestibular hypofunction. Vestibular Rehabilitation. 2007. 3rd ed. Philadelphia: F.A. Davis Co.;333–372.
46. Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res. 1990. 82:167–177.

47. Horak FB. Herdman SJ, editor. Role of the vestibular system in postural control. Vestibular Rehabilitation. 2007. 3rd ed. Philadelphia: F.A. Davis Co.;32–53.
48. Shupert CL, Horak FB, Black FO. Hip sway associated with vestibulopathy. J Vestib Res. 1994. 4:231–244.
49. Brandt T, Strupp M, Benson J. You are better off running than walking with acute vestibulopathy. Lancet. 1999. 354:746.

50. Herdman SJ, Blatt P, Schubert MC, Tusa RJ. Falls in patients with vestibular deficits. Am J Otol. 2000. 21:847–851.
51. Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Res Brain Res Rev. 1989. 14:155–180.

52. Herdman SJ, Clendaniel RA. Herdman SJ, editor. Assessment and interventions for the patient with complete vestibular loss. Vestibular Rehabilitation. 2007. 3rd ed. Philadelphia: F.A. Davis Co.;338–359.
53. Jones TA, Nelson RC. Recovery of vestibular function following hair cell destruction by streptomycin. Hear Res. 1992. 62:181–186.

54. Dieterich M, Brandt T. Imaging cortical activity after vestibular lesions. Restor Neurol Neurosci. 2010. 28:47–56.

55. Norré ME, De Weerdt W. Treatment of vertigo based on habituation. 1. Physio-pathological basis. J Laryngol Otol. 1980. 94:689–696.
56. Norré ME, Beckers A. Vestibular habituation training for positional vertigo in elderly patients. Arch Gerontol Geriatr. 1989. 8:117–122.

57. Brandt T, Daroff RB. Physical therapy for benign paroxysmal positional vertigo. Arch Otolaryngol. 1980. 106:484–485.

58. Das VE, Leigh RJ, Thomas CW, Averbuch-Heller L, Zivotofsky AZ, Discenna AO, et al. Modulation of high-frequency vestibuloocular reflex during visual tracking in humans. J Neurophysiol. 1995. 74:624–632.

59. Lundin-Olsson L, Nyberg L, Gustafson Y. "Stops walking when talking" as a predictor of falls in elderly people. Lancet. 1997. 349:617.

61. Furman JM, Balaban CD, Pollack IF. Vestibular compensation in a patient with a cerebellar infarction. Neurology. 1997. 48:916–920.

62. Telian SA, Shepard NT, Smith-Wheelock M, Kemink JL. Habituation therapy for chronic vestibular dysfunction: preliminary results. Otolaryngol Head Neck Surg. 1990. 103:89–95.

63. Norré ME, Beckers A. Benign paroxysmal positional vertigo in the elderly. Treatment by habituation exercises. J Am Geriatr Soc. 1988. 36:425–429.

64. Brandt T, Dieterich M. Bronstein AM, Brandt T, Woollacott MH, Nutt JG, editors. Postural imbalance in peripheral and central vestibular disorders. Clinical Disorders of Balance, Posture and Gait. 2004. 2nd ed. London: Arnold;146–162.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download