Abstract
Background
Carotid cavernous fistula (CCF) is an abnormal communication between the carotid artery and the cavernous sinus. The pathogenesis of spontaneous CCF remains unclear, although sinus thrombosis is known to be a predisposing factor for dural arteriovenous fistula. Because spontaneous CCFs are mainly of the dural type, we considered that thrombogenic conditions, such as, protein S deficiency might be associated with CCF.
Case Report
A 42-year-old woman complained of conjunctival injection and retro-orbital pain that first appeared 1-month before visiting our hospital. She had no history of head trauma or intracranial surgery. Exophthalmos and chemosis were observed in her left eye, which also had lower visual acuity and higher intraocular pressure than the right eye. Magnetic resonance images and cerebral angiography revealed a left dural CCF. Her protein S was low, at 41% (normal range: 70-140%), but other hematologic values related to coagulation were normal. Her symptoms were relieved after initial transvenous coil embolization. However, a newly developed sixth-nerve palsy was detected 4 days after initial embolization. Follow-up angiography revealed a minimal shunt, and thus transvenous coil embolization was repeated. Two days later, the ophthalmoplegia started reducing, and 1-month later it had almost disappeared.
Conclusions
To the best of our knowledge, this is the first report of spontaneous dural CCF in a Korean patient with concurrent protein S deficiency. Interestingly, transient sixth-nerve palsy developed after transvenous coil embolization in this patient. This additional symptom caused by the residual fistula was relieved after additional transarterial embolization.
Carotid cavernous fistula (CCF) is an abnormal communication between the carotid artery and the cavernous sinus. CCF is classified according to its etiology (traumatic or spontaneous) and anatomy (directly from the carotid artery or from its dural branches).1 Direct CCF is usually traumatic, and dural CCF is usually spontaneous.2 Studies on the factors that predispose to spontaneous CCF are lacking, and its pathogenesis remains unclear.
Protein S is a vitamin-K-dependent plasma protein with an-ticoagulant activity, a cofactor of activated protein C, and an inhibitor of tissue factor pathways during the inactivations of factors Va, VIIa, VIIIa, and Xa.3 The prevalence of protein S deficiency in the general population is less than 0.5%, and it usually occurs due to a heterozygous defect.4 Protein S deficiency might predispose a patient to venous thrombosis, and may be associated with recurrent thromboembolic diseases such as deep vein thrombosis, pulmonary embolism, and cerebral venous thrombosis.5,6
It is known that sinus thrombosis, head trauma, surgery, and hormonal influences are factors that predispose to dural arteriovenous fistula (AVF).7,8 Because spontaneous CCFs are usually of the dural type, we considered that thrombogenic conditions, such as, protein S deficiency, might be related to spontaneous CCF. In this context, we describe a case of spontaneous CCF in a patient with protein S deficiency.
A 42-year-old Korean woman complained of conjunctival injection and a dull retro-orbital pain of 1-month duration in her left eye. Decreased visual acuity of the left eye developed 20-days thereafter, but she did not complain of diplopia. Furthermore, she had no history of head trauma, intracranial surgery, or intake of hormonal drugs. Essential hyper-tension had been detected 6-months previously, and she was taking antihypertensive medication, but her blood pressure was well controlled. Neither the patient nor her family members had any history of thromboembolic disease.
The patient's systolic and diastolic blood pressures were 118 and 76-mmHg, respectively. Exophthalmos, chemosis, and conjunctival injection were observed in her left eye. The visual acuities of her left and right eyes were 20/1,000 and 20/200, respectively, and the intraocular pressures were 25 and 12-mmHg. Light reflexes in both eyes were normal, and no relative afferent papillary defect was observed. Limitations of extraocular motion were not evident and the fundi of both eyes were normal. Ophthalmic bruit was heard on her left eye. Both motor and sensory functions were intact, and deep tendon reflexes were normal. Furthermore, there was no evidence of cerebellar dysfunction, and she did not have an abnormal gait.
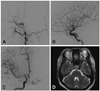
Magnetic resonance and cerebral angiography images revealed a left CCF that was fed by the bilateral internal and external carotid arteries (dural CCF) (Fig. 1). The CCF drain-ed into the left superior ophthalmic vein and the left sphenoparietal sinus. No thrombosis was observed in the cavernous sinus. Hematologic tests showed normal levels of protein C and antithrombin III. Factor V Leiden and antiphospholipid antibody were not detected. Her protein S level was low at 41% (normal range: 70-140%).
The cavernous sinus was embolized via a transvenous approach using 19 coils, and the fistula was completely obliterated. Chemosis, conjunctival injection, and orbital pain were relieved after embolization, and the patient's visual acuity improved from 20/1,000 to 20/100, and the intraocular pressure decreased from 25 to 17-mmHg.
Sixth-nerve palsy with orbital pain developed suddenly 4 days after the initial embolization; the intraocular pressures in the left and right eyes were 17 mmHg and 12 mmHg, respe-ctively. Follow-up angiography revealed a minimal shunt from the dural branch of the left middle meningeal artery. Thus, transarterial glue injection and second coil embolization were performed via a transfacial vein, which resulted in completely obliteration of the shunt. Anticoagulation therapy was then started to prevent additional venous thrombosis due to the protein S deficiency. Two days after the second embolization (6-days after the initial embolization) diplopia and orbital pain started diminishing, and at 1-month after embolization, only minimal diplopia in the extreme left lateral gaze remained.
We report a case of spontaneous dural CCF and concurrent protein S deficiency. Several studies have shown that dural AVF is associated with thrombosis of the involved sinus.9,10 One possible mechanism for the development of acquired dural AVF is that increased venous pressure after sinus thrombosis opens intrinsic channels between the cerebral arteries and venous sinuses.11 Another is that increased venous pressure may elicit local ischemia and increase ischemia-related angiogenesis.12 Accordingly, we considered that dural AVF might be associated with thrombogenic conditions such as protein S deficiency, resulting in the development of sinus thrombosis.
A few retrospective studies have investigated the association between dural AVF and thrombogenic conditions.13-15 A German study found that the prevalence of the G20210A mutation was higher in patients with spontaneous dural AVF than in a control group, but only one patient had protein S deficiency.14 In a recent Japanese study, 4 (22.2%) of 18 patients with spontaneous dural CCF were found to have protein S deficiency, but this did not reach statistical significance15, which was attributed to the small sample size and the prevalence of protein S deficiency being higher in the Japanese control group than in Caucasians. However, no report of an association between spontaneous dural AVF and thrombogenic conditions has been issued in any other country.
Sinus thrombosis is considered to play an important role in the development of dural AVF in patients with thrombogenic conditions. Interestingly, our patient did not have sinus thrombosis. However, dural AVF and sinus thrombosis might not always develop simultaneously.15 There is a report of dural AVF developing more than 12-months after sinus thrombosis.16 It is possible that the inciting thrombosis had already resolved when the CCF was detected.
In our patient a new sixth-nerve palsy developed after the initial embolization. In a previous study, six cases (10.7%) developed cranial nerve signs after transvenous embolization, including sixth-nerve palsy.17 In most cases, overpacking of the cavernous sinus probably caused the transient cranial nerve symptoms; progressive thrombosis of the cavernous sinus and direct injury of the nerve by a coil or micro-wire/microcatheter are also possible causes.18,19 Although a recent study measured coil volumes and locations, a method devised to demonstrate coil overpacking was not feasible.20 Thus, it is unclear whether the cause of newly developed sixth nerve palsy in our case was overpacking, a residual shunt, or progressive thrombosis related to protein S deficiency. The disappearance of symptoms after the second embolization and the intraocular pressure being higher in the left eye than in the right eye after embolization and anticoagulation support the theory of residual shunt or progressive thrombosis theories, but the overpacking theory is supported by the following findings: 1) The size of the newly developed shunt was negligible compared to that of the initial CCF. 2) The intraocular pressure of left eye (17 mmHg) was higher than that of the right eye, which was within normal limit. 3) The newly developed cranial nerve palsies caused by overpacking in previously described cases disappeared spontaneously.19
To our knowledge, this is the first report of spontaneous CCF in a Korean patient with concurrent protein S deficiency. Few studies have investigated the association between CCF and thrombogenic conditions, which makes further adequately powered studies necessary. Interestingly, a transient sixth-nerve palsy developed after transvenous coil embolization, but this additional symptom-which was caused by residual fistula-was relieved after additional transarterial embolization.
Figures and Tables
Fig. 1
Cerebral angiography images show a left CCF fed by the bilateral internal and external carotid arteries. A: Lateral arteriogram of left external carotid artery. B: Lateral arteriogram of left internal carotid artery. C: Anteroposterior arteriogram of right internal carotid artery. D: Magnetic resonance images indicate a prominent superior ophthalmic vein. CCF: carotid cavernous fistula.
References
1. Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985. 62:248–256.

2. Uehara T, Tabuchi M, Kawaguchi T, Mori E. Spontaneous dural carotid cavernous sinus fistula presenting isolated ophthalmoplegia: evaluation with MR angiography. Neurology. 1998. 50:814–816.

3. Castoldi E, Hackeng TM. Regulation of coagulation by protein S. Curr Opin Hematol. 2008. 15:529–536.

4. Dykes AC, Walker ID, McMahon AD, Islam SI, Tait RC. A study of Protein S antigen levels in 3788 healthy volunteers: influence of age, sex and hormone use, and estimate for prevalence of deficiency state. Br J Haematol. 2001. 113:636–641.

5. Comp PC, Esmon CT. Recurrent venous thromboembolism in patients with a partial deficiency of protein S. N Engl J Med. 1984. 311:1525–1528.

6. Kim JS, Jeong SH, Kim DH, Kim J. Safety and feasibility of subcutaneous low molecular weight heparin for cerebral venous sinus thrombosis. J Clin Neurol. 2005. 1:134–141.

8. Suh DC, Lee JH, Kim SJ, Chung SJ, Choi CG, Kim HJ, et al. New concept in cavernous sinus dural arteriovenous fistula: correlation with presenting symptom and venous drainage patterns. Stroke. 2005. 36:1134–1139.

9. Handa J, Yoneda S, Handa H. Venous sinus occlusion with a dural arteriovenous malformation of the posterior fossa. Surg Neurol. 1975. 4:433–437.
10. Haisa T, Yoshida S, Ohkubo T, Yoshikawa K, Machida T. Primary empty sella in association with superior sagittal sinus thrombosis and dural arteriovenous malformation. Case report. J Neurosurg. 1994. 81:122–125.

11. Yassari R, Jahromi B, Macdonald R. Dural arteriovenous fistula after craniotomy for pilocytic astrocytoma in a patient with protein S deficiency. Surg Neurol. 2002. 58:59–64. discussion 64.

12. Kojima T, Miyachi S, Sahara Y, Nakai K, Okamoto T, Hattori K, et al. The relationship between venous hypertension and expression of vascular endothelial growth factor: hemodynamic and immunohistochemical examinations in a rat venous hypertension model. Surg Neurol. 2007. 68:277–284. discussion 284.

13. Gerlach R, Yahya H, Rohde S, Böhm M, Berkefeld J, Scharrer I, et al. Increased incidence of thrombophilic abnormalities in patients with cranial dural arteriovenous fistulae. Neurol Res. 2003. 25:745–748.

14. Gerlach R, Boehm-Weigert M, Berkefeld J, Duis J, Raabe A, Seifert V, et al. Thrombophilic risk factors in patients with cranial and spinal dural arteriovenous fistulae. Neurosurgery. 2008. 63:693–698. discussion 698-699.

15. Izumi T, Miyachi S, Hattori K, Iizuka H, Nakane Y, Yoshida J. Thrombophilic abnormalities among patients with cranial dural arteriovenous fistulas. Neurosurgery. 2007. 61:262–268. discussion 268-269.

16. Preter M, Tzourio C, Ameri A, Bousser MG. Long-term prognosis in cerebral venous thrombosis. Follow-up of 77 patients. Stroke. 1996. 27:243–246.
17. Kim DJ, Kim DI, Suh SH, Kim J, Lee SK, Kim EY, et al. Results of transvenous embolization of cavernous dural arteriovenous fistula: a single-center experience with emphasis on complications and management. AJNR Am J Neuroradiol. 2006. 27:2078–2082.
18. Klisch J, Schipper J, Husstedt H, Laszig R, Schumacher M. Transsphenoidal computer-navigation-assisted deflation of a balloon after endovascular occlusion of a direct carotid cavernous sinus fistula. AJNR Am J Neuroradiol. 2001. 22:537–540.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download