Abstract
Background
The main treatment for acute arterial ischemic stroke is intravenous or intra-arterial thrombolysis within a particular time window. Endovascular mechanical embolectomy is another treatment option in the case of major artery occlusion. Endovascular mechanical embolectomy is a useful technique for restoring blood flow in patients with large-vessel occlusion, and especially in those who are contraindicated for thrombolytics or in whom thrombolytic therapy has failed.
The treatment options for acute ischemic stroke vary depending on the time of onset, imaging findings, and type of stroke. In adults with acute arterial ischemic stroke, outcome is improved by administering intravenous recombinant tissue plasminogen activator (rt-PA) within 3 hours of symptom onset or by applying intra-arterial thrombolysis with recombinant prourokinase within 6 hours of stroke onset caused by occlusion of the middle cerebral artery (MCA).1-3 The administration of intravenous rt-PA is the only proven effective treatment for ischemic stroke.4
Microsurgical embolectomy has been performed sporadically for acute MCA occlusion since the late 1950s.5-9 However, emergency microsurgical embolectomy has not been widely performed since the introduction of local intra-arterial thrombolysis. Endovascular embolectomy was recently introduced for the treatment of major artery occlusion, and there are several reports of its efficacy.10,11 However, a low rate of recanalization and a high mortality rate have been noted in the cases of acute occlusion of the distal intracranial segment of the internal carotid artery (ICA), even when endovascular treatment is used.12 Moreover, both intravenous and intra-arterial thrombolytic therapies are contraindicated in some cerebral infarctions combined with extra-axial hemorrhage (e.g., subdural hematoma).
We report herein two cases of emergency microsurgical embolectomy for the treatment of acute MCA and ICA occlusion as an alternative treatment for major artery occlusion.
A 75-year-old man was admitted with sudden aphasia and right hemiplegia. His score on the National Institutes of Health Stroke Scale (NIHSS) at admission was 18. The time between the onset of symptoms and admission was 30 minutes. The medical history of this patient showed that he had been admitted to our hospital 5 months previously because of left MCA territory infarction. At that time, cerebral angiography revealed a left cervical ICA stenosis with intraluminal atheroma (Fig. 1A). The patient was later discharged against stent recommendation. Brain CT scans performed at the same time revealed a high-density spot at the carotid and Sylvian cistern and no abnormalities except an old localized cerebral infarction in the left temporoparietal lobe along the MCA territory (Fig. 1B, C).
After ruling out intracerebral hemorrhage, a full dose of rt-PA (63 mg) was administered as a continuous infusion; the door-to-needle time was 40 minutes. After administering rt-PA, the patient's NIHSS score was 17. Subsequent MRI revealed an acute cerebral infarction in the left MCA territory with a perfusion-diffusion mismatch (Fig. 1D, E). Cerebral angiography revealed an embolic left terminal ICA "T" occlusion (Fig. 1F). Intra-arterial mechanical and chemical thrombolysis (urokinase, 200,000 U) for 40 minutes did not provide recanalization. Since thrombolysis and endovascular recanalization failed and there was sufficient time to restore the cerebral circulation by microsurgical embolectomy, which must be performed within 8 hours after the onset of symptoms, we decided to perform that procedure. The preoperative NIHSS score was 16.
The patient underwent microsurgical embolectomy via a left pterional approach 30 minutes after the interventional procedure. A left ICA occlusion was confirmed in the operative field (Fig. 2A). After arteriotomy, the embolus was removed (Fig. 2B). The cerebral circulation was restored at 6.5 hours after the onset of symptoms. The patient's postoperative NIHSS score was 12. Postoperative angiography revealed recanalization of the left ICA occlusion and mild M1 stenosis at the arteriotomy site (Fig. 2C). After stent placement in the left cervical proximal ICA, the patient was discharged with left hemiparesis grade 4+. The NIHSS scores at discharge and 3 months postoperatively were 9 and 4, respectively.
A 74-year-old woman presented with left hemiplegia. The time between the onset of symptoms and admission was 3.5 hours. A swelling at the left frontotemporal area was observed on physical examination, suggesting that the patient had experienced a head trauma during the hemiplegic attack. On neurological examination, her NIHSS score was 20. There were no abnormal findings on CT. Cerebral angiography revealed a right M2 occlusion (Fig. 3A). Subsequently, a microcatheter was introduced into the MCA, through which urokinase was injected. The M2 branch was recanalized (Fig. 3B). After thrombolysis, the left hemiplegia improved and the NIHSS score was 8.
A follow-up brain CT scan showed an acute subdural hematoma at the left frontotemporoparietal area with a mild midline shift (Fig. 3C). The patient was treated conservatively. On the ninth hospital day, the patient complained of left motor weakness and dysarthria; her NIHSS score was aggravated to 16. A brain CT scan revealed a resolving subdural hematoma, and CT angiography revealed a right MCA occlusion (Fig. 4A). Because of the previous hemorrhage, we performed emergency microsurgical embolectomy. The MCA occlusion was confirmed in the operative field, and the embolus was removed (Fig. 4B, C). The cerebral circulation was restored 4 hours after the onset of symptoms. The postoperative course was uneventful, but the left motor weakness did not improve. The patient's postoperative NIHSS score was 13. Follow-up brain MRI and CT angiography revealed a small, right periventricular infarction and recanalization of the MCA (Fig. 4D, E). Her NIHSS scores at discharge and 3 months postoperatively were 11 and 10, respectively.
Intra-arterial thrombolysis is an option for recanalization therapy in selected patients who are not candidates for intravenous rt-PA. Despite the uncontrolled observation that recanalization rates may be higher with intra-arterial thrombolysis than with intravenous thrombolysis, the clinical benefit may be counter-balanced by delays in treatment initiation using the intra-arterial approach. The intra-arterial approach has been promoted because it can deliver a high concentration of thrombolytic agents into the thrombus.13 Patients who are evaluated within 6 hours of symptom onset but who are ineligible to receive intravenous thrombolysis because of recent surgery or other procedures may be candidates for intra-arterial thrombolysis.3
Endovascular mechanical embolectomy is another treatment option for major artery occlusion. Mechanical embolectomy is a useful technique for restoring blood flow in patients with large-vessel occlusion, and especially in those who are ineligible for thrombolytics or in whom thrombolytic therapy has failed.
Patients who present beyond 3 hours after stroke or who are ineligible to undergo intravenous or intra-arterial thrombolysis due to intracranial hemorrhage, as in our case 2, cannot receive recanalization therapy. In addition, acute distal ICA occlusion has a low rate of recanalization, even with endovascular treatment, as in our case 1. Conventional thrombolytic therapy for occlusion of the carotid bifurcation may be ineffective because of the low recanalization rate.15 The mean rate of recanalization for ICA occlusion after thrombolytic therapy has been reported to be only 11%.12 In such cases, the authors suggest that microsurgical embolectomy within 8 hours of onset of stroke is an alternative treatment option for major artery occlusion. The authors' rationale for microsurgical embolectomy is based on the inclusion criteria (patients with large-vessel stroke treated within 8 hours of symptom onset and patients with persistent large-vessel occlusion after intravenous rt-PA treatment) and the results of the Multi Mechanical Embolus Removal in Cerebral Ischemia trial.14
Previous studies indicated that the recanalization rate of microsurgical embolectomy was 100%.12,16 Horiuch et al. reported16 a postoperative hemorrhagic infarction rate of 8.3%. Patient outcomes were as follows: good recovery, 41.7%; moderate disability, 16.7%; severe disability, 25%; vegetative state, 8.3%; and death due to acute heart failure, 8.3%. Surgery-related complication rates are also acceptable.2,10,11,14
Microsurgical embolectomy can be safely performed with less morbidity for several reasons even after intravenous or intra-arterial thrombolytic therapy. First, microsurgical embolectomy is not performed through the neural tissue but rather through the subarachnoid space. The risk of procedure-related brain parenchymal hemorrhage is therefore very low. Second, recent advances in microsurgical techniques have made it possible to perform microsurgical embolectomy safely. Third, the conventional large skin and bone flap is not necessary in this procedure; microsurgical embolectomy can be performed through a minimal skin incision and craniotomy. For these reasons, microsurgical embolectomy can be performed safely and with less surgery-related morbidity (especially bleeding). Therefore, the authors suggest that emergency microsurgical embolectomy is an another option in selected acute ischemic stroke patients with distal ICA "T" occlusion or in patients who are ineligible to receive intravenous or intra-arterial thrombolysis.
In conclusion, emergency microsurgical mechanical embolectomy may be an alternative option for restoring blood flow in selected patients with large-vessel acute ischemic stroke, and especially in those who are ineligible for thrombolytics or in whom thrombolytic therapy has failed. Clinicians should be aware that microsurgical embolectomy can be performed in selected cases only when the time elapsed from the onset of symptoms to reopening of the occluded vessel is less than 8 hours. Further well-designed clinical trials are needed to address the clinical effectiveness of microsurgical embolectomy.
Figures and Tables
Fig. 1
Case 1. A: Cerebral angiography 5 months prior to the most recent admission showing left cervical internal carotid artery (ICA) stenosis with intraluminal atheroma (arrowhead). B and C: Admission brain CT scans demonstrating a high-density spot at the carotid and Sylvian cistern (arrow) and revealing no abnormalities except for an old localized cerebral infarction in the left temporoparietal lobe along the middle cerebral artery (MCA) territory. D and E: Diffusion and perfusion brain MRI showing diffusion-perfusion mismatch with the finding of acute cerebral infarction and delay of time to peak in the left MCA territory. F: Left ICA angiogram, anteroposterior view, disclosing a left terminal ICA occlusion ("T" occlusion).
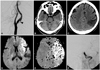
Fig. 2
Case 1. A and B: Intraoperative photographs showing the occluded terminal ICA. Asterisk indicates the embolus; arrowhead, arteriotomy site. C: Postoperative left ICA angiogram, anteroposterior view, revealing successful ICA recanalization and mild M1 stenosis at the arteriotomy site (arrow). ICA: internal carotid artery.

Fig. 3
Case 2. A: Right ICA angiogram, anteroposterior view, showing a right M2 occlusion. B: Right ICA angiogram, after injection of urokinase, revealing recanalization of the M2 branch. C: Follow-up brain CT scan demonstrating an acute subdural hematoma at the left frontotemporoparietal area with a mild mass effect. ICA: internal carotid artery.
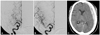
Fig. 4
Case 2. A: Brain CT angiography (CTA) on the ninth hospital day revealing a right MCA occlusion. B and C: Intraoperative photographs showing the occluded MCA. Asterisk indicates the embolus. D: Postoperative brain diffusion MRI showing a high signal intensity at the right periventricular area (arrow). E: Follow-up CTA showing sufficient blood flow in the right MCA.

References
1. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995. 333:1581–1587.
2. Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Prolyse in Acute Cerebral Thromboembolism. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. JAMA. 1999. 282:2003–2011.

3. Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American heart association/American stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council, and the atherosclerotic peripheral vascular disease and quality of care outcomes in research interdisciplinary working groups: the American academy of neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007. 38:1655–1711.

4. Choi JC, Kang SY, Kang JH, Ko YJ, Bae JM. Are in-Hospital delays important obstacles in thrombolytic therapy following acute ischemic stroke? J Clin Neurol. 2007. 3:71–78.

5. Gagliardi R, Benvenuti L, Guizzardi G. Acute operation in cases of middle cerebral artery occlusion. Neurosurgery. 1983. 12:636–639.

6. Garrido E, Stein BM. Middle cerebral artery embolectomy. Case report. J Neurosurg. 1976. 44:517–521.
7. Khodadad G. Middle cerebral artery embolectomy and prolonged widespread vasospasm. Stroke. 1973. 4:446–450.

8. Meyer FB, Piepgras DG, Sundt TM Jr, Yanagihara T. Emergency embolectomy for acute occlusion of the middle cerebral artery. J Neurosurg. 1985. 62:639–647.

9. Welch K. Excision of occlusive lesions of the middle cerebral artery. J Neurosurg. 1956. 13:73–80.

10. Gobin YP, Starkman S, Duckwiler GR, Grobelny T, Kidwell CS, Jahan R, et al. MERCI 1: a phase 1 study of Mechanical Embolus Removal in Cerebral Ischemia. Stroke. 2004. 35:2848–2854.
11. Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005. 36:1432–1438.

12. Touho H, Morisako T, Hashimoto Y, Karasawa J. Embolectomy for acute embolic occlusion of the internal carotid artery bifurcation. Surg Neurol. 1999. 51:313–320.

13. Qureshi AI. Endovascular treatment of cerebrovascular diseases and intracranial neoplasms. Lancet. 2004. 363:804–813.

14. Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008. 39:1205–1212.
15. Jansen O, von Kummer R, Forsting M, Hacke W, Sartor K. Thrombolytic therapy in acute occlusion of the intracranial internal carotid artery bifurcation. AJNR Am J Neuroradiol. 1995. 16:1977–1986.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download