Abstract
Background
Numerous neck muscles are involved in neck movements, and so isolated neck weakness is extremely uncommon in cerebral infarction.
Case Report
We report herein the case of a 65-year-old woman with hypertension and acute cortical infarction, presenting with ipsilateral head tilt and contralateral sensory changes in the neck and shoulder area, which has never been described before.
Conclusions
Transient neck weakness and sensory deficits can occur in acute cortical infarction. The motor representation of the neck muscles can be at the same level of the cortical sensory representation, near to the level of the trunk representation, which is in contrast to Penfield's findings. Several possible mechanisms for the ipsilateral tilt are described.
A small cortical infarction can produce isolated motor weakness, which usually manifests in the extremities.1-3 Isolated neck weakness in cerebral infarction is very rare, probably because numerous muscle groups with functional overlap are involved in producing various neck movements. For example, the neck flexors are the sternocleidomastoid (SCM), platysma, suprahyoid, infrahyoid, scalene complex, and the prevertebral group of muscles.4 Clinically, the only muscles that can be examined individually are the SCM and trapezius, and hence neck weakness is evaluated by gross movements such as neck flexion and extension, rather than by examining particular muscles.4
We experienced a patient exhibiting ipsilateral lateral tilt of the neck and contralateral hypesthesia in the neck and shoulder area due to a tiny precentral gyrus lesion, which has never been reported previously. Several possible mechanisms for the ipsilateral tilt are discussed.
A 65-year-old, left-handed woman with hypertension was admitted for the evaluation of acute transient neck lateral bending to the right lateral side, and mild hypesthesia on her left lower neck, shoulder, and upper back areas. Ten days before admission she felt a slight transient lateral flexion of her neck (from vertical to 10-20°) when in the resting position. She denied any other direction of head tilt, such as to the right anterolateral side, or any increased tension in her neck muscles suggesting dystonia or seizure.
Three days before admission the transient tilting recurred and became more frequent. Tingling and numbness developed around the left upper back, shoulder, and upper arm. Her family history was unremarkable. A neurological examination revealed normal motor function and decreased touch sensation on her left neck and shoulder areas (Fig. 1A). Pinprick and temperature sensations were normal. Brain magnetic resonance imaging (MRI) was performed. Diffusion-weighted-imaging and apparent diffusion coefficient revealed an acute focal infarct, high up on the cerebral convexity in the right precentral cortex (Fig. 1B). Magnetic resonance angiography, routine laboratory tests, electrocardiography, and transthoracic echocardiography all yielded normal results. Electroencephalography showed no epileptiform activity.
This patient was treated with antiplatelet medication. Eight days later the sensory deficits had completely resolved, and no recurrence of the symptoms occurred during an 18-month follow-up period.
Described herein is a rare case of isolated neck tilt showing ipsilateral neck lateral flexion and contralateral sensory deficits. Given the close temporal relationship between the symptoms and the acute lesion location, the lesion may be associated with the cortical representation of the neck muscles. Brain MRI revealed only one acute lesion with a high signal intensity on Diffusion-weighted-imaging and a low signal intensity on apparent diffusion coefficient; since we performed the MRI soon after symptom onset, we believe that this was the most likely underlying lesion.
Cortical representation is defined as the motor and sensory function of specific body parts, and is represented in specific cortical regions, as first suggested by Hughlings Jackson.5 Conventionally, the motor and sensory cortical representations of the neck were not believed to reside at the same level in the cerebral cortex, as noted in the well-known study of Penfield.6 However, an electrophysiological study demonstrated that the motor cortical area was close to the trunk representation, at a comparable level to the sensory cortical representation of the neck,7 similar to our observation. Penfield's finding of the different levels of the motor and sensory representations of the neck appears to defy clinical intuition, because most other cortical motor-sensory representations described in his study were at the same level. It seems that neck movement and sensation analysis in his study was not conclusive because of data limitations.
Potential limitations of our observations should be noted. We were unable to document a specific muscle weakness, with no weakness being evident on neurological examination; this could limit the clinical interpretation. As mentioned above, it is difficult to examine individual neck muscles in the clinical setting. More precise information with a motion analysis device for this patient's neck position may be helpful.8,9 Without objective information about the involved muscles, several explanations for her neck weakness are possible. The SCM, splenius capitis, scalene complex, and splenius cervicis muscles are involved in neck lateral flexion, and these individual muscles have several shared actions.10
Our observations could have been due to involvement of the ipsilateral SCM, which is the predominant muscle in neck. The ipsilateral weakness pattern has been reported previously in association with supranuclear lesions in hemiparetic patients.11-13 However, contradictory results had led to debate about whether the SCMs receive ipsilateral, contralateral, or bilateral cortical innervations.7,11-14 In our case it was not clear whether the patient's head tilt could be attributed to SCM weakness, because she did not complain of any weakness of head turning. Moreover, selective SCM paresis does not usually cause changes in the head position in the resting position.4
Another explanation is that this patient's neck weakness was attributable to the splenius capitis and scalene complex on the contralateral side to the cerebral lesion. If the splenius capitis and scalene complex for lateral bending on the contralateral side to the cerebral lesion are weak, head tilt may be toward the lesion side because of the relatively hyperactive splenius capitis of the lesion side.
Another argument can be made for her sensory deficit in precentral gyrus lesion, but the somatosensory cortical area is not restricted to the postcentral gyrus15 and electrical stimulation of the precentral gyrus can induce sensory symptoms.6 Many researchers do not concern the sensory symptoms in stroke. A detailed sensory examination showed that most patients with pure motor stroke have a sensory abnormality.16
In conclusion, the case presented herein is a rare one wherein the ipsilateral neck tilt and contralateral sensory deficit were due to a focal cortical infarction in an area relating to the neck muscles. The motor representation of neck muscles may be at the same level as the sensory representation, near to the level of the trunk representation, which is consistent with recent electrophysiological findings.7
Figures and Tables
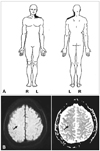 | Fig. 1A: Schematic diagram of the decreased sensation of the patient's neck and shoulder areas. B: Brain diffusion-weighted magnetic resonance imaging and apparent diffusion coefficient map showing a tiny, acute infarction located high up on the cerebral convexity in the right precentral cortex (arrow), a comparable level to the sensory representation of the neck in the postcentral cortex. |
References
1. Han YS, Ha SW, Cho JS, Park SE, Kim JM, Han JH, et al. Isolated weakness of middle, ring, and little fingers due to a small cortical infarction in the medial precentral gyrus. J Clin Neurol. 2006. 2:146–148.

2. Ku BD, Lee EJ, Kim H. Cerebral infarction producing sudden isolated foot drop. J Clin Neurol. 2007. 3:67–69.

3. Nah HU, Park HK, Kang DW. Isolated shoulder weakness due to a small cortical infarction. J Clin Neurol. 2006. 2:209–211.

4. Campbell WW. The spinal accessory nerve, Motor strength and power. DeJong's The Neurologic Examination. 2005. 6th ed. Philadelphia: Lippincott Williams & Wilkins;263–269. 343–394.
5. Jackson JH. Selected writings of John Hughlings Jackson. 1931. London: Hodder and Stoughton;37–76.
6. Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937. 60:389–443.

7. Thompson ML, Thickbroom GW, Mastaglia FL. Corticomotor representation of the sternocleidomastoid muscle. Brain. 1997. 120:245–255.

8. Boccagni C, Carpaneto J, Micera S, Bagnato S, Galardi G. Motion analysis in cervical dystonia. Neurol Sci. 2008. 29:375–381.

9. Strimpakos N, Sakellari V, Gioftsos G, Papathanasiou M, Brountzos E, Kelekis D, et al. Cervical spine ROM measurements: optimizing the testing protocol by using a 3D ultrasound-based motion analysis system. Cephalalgia. 2005. 25:1133–1145.

10. Clement CD. Gray's Anatomy of the Human Body. 1985. 30th ed. Philadelphia: Lea & Febiger.
11. Hayward R. Observations on the innervation of the sternomastoid muscle. J Neurol Neurosurg Psychiatry. 1986. 49:951–953.

12. Manon-Espaillat R, Ruff RL. Dissociated weakness of sternocleidomastoid and trapezius muscles with lesions in the CNS. Neurology. 1988. 38:796–797.

13. Mastaglia FL, Knezevic W, Thompson PD. Weakness of head turning in hemiplegia: a quantitative study. J Neurol Neurosurg Psychiatry. 1986. 49:195–197.

14. DeToledo JC, Dow R. Sternomastoid function during hemispheric suppression by amytal: insights into the inputs to the spinal accessory nerve nucleus. Mov Disord. 1998. 13:809–812.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download