Abstract
Background and Purpose
There is recent evidence of various types of morphological changes in the hippocampus of a rodent model of medial temporal lobe epilepsy (mTLE). However, little is known about such changes in humans. We examined the histological changes [i.e., neuronal loss, cell genesis, and granule cell dispersion (GCD)] in surgical hippocampal specimens taken from patients with mTLE.
Methods
Nissl staining, and nestin and Prox1 immunohistochemistry were performed on human hippocampal specimens obtained from patients with medically intractable mTLE, thus allowing the analysis of neuronal loss, cell genesis, and GCD, respectively. We also assessed the correlations between clinical parameters and the histopathologic findings.
Results
The degree of cell genesis in the granule cell layer was significantly correlated with the severity of GCD, history of childhood febrile seizures, and frequent generalized seizures. Cell genesis was not correlated with cell death, age at seizure onset, duration of epilepsy, or the mean frequency of all seizures.
Hippocampal sclerosis (HS) is the most common lesional abnormality identified in patients with temporal lobe epilepsy (TLE).1,2 HS is characterized by severe loss of the principal neurons in areas CA1 and CA3 of the hippocampus, and is frequently associated with widening of the granule cell layer of the dentate gyrus, termed granule cell dispersion (GCD), which is observed in about 40-50% of surgical temporal lobe specimens.3-6 Despite their importance in TLE, the pathogenic mechanisms underlying this distinctive hippocampal pathology have not yet been identified. Whether HS represents the cause or the consequence of chronic seizure activity and pharmacoresistant TLE also remains to be established.7
The clinical significance of the morphological changes observed in the hippocampi of patients with medial TLE (mTLE) is ambiguous, in spite of the many relevant studies that have been conducted over the last 50 years. Although there have been many suggestions of a positive correlation between the severity of hippocampal neuronal loss and clinical parameters that indicate a greater seizure burden [e.g., younger age of onset, longer duration of epilepsy, greater number of generalized seizures, and presence of status epilepticus (SE) and initial precipitating injuries (IPI)], there is currently no reliable evidence of such a relationship.8-13
In addition to the relationship between clinical and pathological parameters, there is no established evidence of a relationship between the pathological parameters themselves. For example, the development of GCD in the hippocampus is thought to be affected by excessive neurogenesis as a result of seizures in rodent models of TLE.14 However, there are insufficient and conflicting data about the relationship between GCD and cell proliferation in the human hippocampus.15,16
In the study presented here, we examined the histological changes associated with TLE in terms of neuronal loss, cell genesis, and GCD in surgical hippocampal specimens taken from patients with pharmacoresistant mTLE and HS. We also examined both the clinicopathologic relationship and the interrelationship between these pathologic changes to better understand the clinical significance and pathologic mechanisms underlying the histological changes in mTLE.
Cases were selected from the pathology archives at the Departments of Neurology and Neurosurgery, Seoul National University Hospital, Seoul, Korea. The research was approved by the Hospital Committee on Human Ethics. The hippocampal specimens were obtained from 26 patients who had undergone temporal lobectomy between 2000 and 2006, including hippocampal resection, for the treatment of medically intractable mTLE with HS. Medically intractable patients were defined as those whose seizures were poorly controlled with two or more anticonvulsant drugs prescribed by an epileptologist, including at least one of the following: phenytoin, carbamazepine, and valproic acid.17 All patients went through a comprehensive clinical, electrophysiological, neuropsychological, and imaging evaluation before the epilepsy surgery.
Clinical data were obtained for each patient by reviewing all records available in the electronic medical records at the hospital. These data included the patient's sex, age at epilepsy onset, age at surgery, duration of epilepsy, average frequency of preoperative complex partial and generalized seizures, history of childhood febrile seizure (FS) or other significant IPI,18 age at FS or IPI, history of SE, and surgical outcomes.
The brain specimens were cryopreserved as tissue blocks for cryostat sectioning at a thickness of 7 µm. Coronal sections were taken from the right or left hippocampus, and every seventh section of the hippocampus (6 sections per specimen) was subjected to semiquantitative immunohistochemical analysis. All hippocampal specimens were processed identically to minimize the effect of differential shrinkage on neuronal density measurements. Histological evaluations were performed as described previously.19-21 Paraffin-embedded sections from selected HS patients were dewaxed, rehydrated through graded alcohols, and taken to water. Sections were microwaved for 15 minutes in 0.05 M EDTA (pH 7.5) and then allowed to cool for 20 minutes.
Sections were stained using the Nissl method and labeled with antinestin (1 : 100, Chemicon, Temecula, CA, USA) and anti-Prox1 antibodies (1 : 60, Abcam, Cambridge, UK). Nestin, which is a protein of the intermediate filament family, is typical of undifferentiated neural stem and progenitor cells.22 Prox1 is expressed in postmitotic dentate granule cells, is specific for this cell type in the adult rat dentate gyrus,23-25 and is used to identify ectopic granule cells following pilocarpine-induced SE.26,27 Biotinylated goat antimouse IgG (ABC, Sigma, Poole, UK) was used as the secondary antibody. The biotin signal was detected using 3,3'-diaminobenzidine (brown) and Vector VIP (purple). Prox1 expression was detected by nickel-enhanced diaminobenzidine peroxidase immunohistochemistry, as described previously.26,27 We also examined the extrahippocampal pathological findings. The surgically excised temporal lobe specimens were fixed in formaldehyde after extirpation and then stained with hematoxylin and eosin.
Histological analysis was performed in the predefined areas of consecutive coronal blocks of 6 serial sections. The cells of interest were evaluated in the CA3 area for hippocampal neuronal damage, the hilus for nestin immunoreactivities, and the granule cell layer for GCD. The region of interest was set to include the proximal, middle, and distal subfields of area CA3 stratum pyramidale (magnification ×400), the whole hilar area (magnification ×400), and the superior and inferior portions of the granule cell layer (magnification ×600). It is not possible to perform unbiased stereology in human surgical tissue samples where the entire region of interest is not present,28 and so we adapted the semiquantitative scoring system of histopathological results as follows. Neuronal loss was measured on a scale of 0-3, where grades 0, 1, 2, and 3 corresponded to 0%, 20%, 20-50%, and >50% of the neurons lost in the particular subfield, respectively. The mean and SD values of the score were calculated from the scored sections.28 Cell genesis was measured on a scale of 1-4, where grade 1 was "few or no nestin-positive cells present (<3 cells/high-power field, HPF)", grade 2 was "a few nestin-positive cells present (3-10 cells/HPF)", grade 3 was "a moderate number of nestin-positive cells present (10-100 cells/HPF)", and grade 4 was "a high number of nestin-positive cells present (>100 cells/HPF)." The mean and SD values were calculated from the scored sections for each area separately (modified from Pirttilä et al.28). GCD was also measured on a scale of 1-4, where grade 1 describes epileptic qualities and a nondispersed appearance, grade 2 describes granule cell bodies dispersed into the molecular layer forming an irregular outer border, grade 3 describes double-layer granule cell bodies organized into two layers, and grade 4 describes predominantly granule cell death (Fig. 1).3,28
The data are presented as mean±SD values. The Pearson correlation coefficient was determined to assess the associations between variables. Two groups were compared by chi-square analysis with arbitrary dichotomization, using SPSS for windows (version 15.0; SPSS Inc, Chicago, IL, USA). A value of p<0.05 was considered significant.
The clinical characteristics of the 26 patients, who comprised 15 men and 11 women who were aged 32.07±9.29 years (range, 16-43 years) at the time of surgery, are listed in Table 1. The age at the onset of epilepsy was 11.11±6.98 years (range, 1-27 years), and the duration of epilepsy at the time of collection was 17.76±10.58 years (range, 1-42 years). All patients had complex partial seizures, and the frequency of seizures per month was 4.25±5.98 (range, 0.5-30). In 16 patients (61.5%), secondary generalization frequently occurred more than once per month. A history of childhood FS was reported in 13 patients (50%), and 7 patients reported other relevant causative childhood events (IPI), for example head trauma, meningitis, encephalitis, and Reye syndrome. No patient reported a history of SE. Most of the patients (80.7%) became seizure-free after their epilepsy surgery.
The pathological grading scores of Nissl (for neuronal loss), nestin (for cell genesis), and Prox1 (for GCD) are listed in Table 2, based on the grading system presented in Fig. 1. Most patients showed a moderate-to-severe amount of neuronal loss: grade 3 in 12 patients (46.2%), grade 2 in 10 patients (38.5%), and grade 1 in 1 patient (3.8%). Most patients showed a mild-to-moderate amount of cell genesis: grade 1 in 8 patients (30.8%), grade 2 in 6 patients (23.1%), grade 3 in 11 patients (42.3%), and grade 4 in 1 patient (3.8%). All patients exhibited some degree of GCD: grade 2 in 13 patients (50%), grade 3 in 7 patients (26.9%), and grade 4 in 6 patients (23.1%). With regard to the extrahippocampal pathological findings, only one patient exhibited apparent cortical dysplasia on magnetic resonance imaging (MRI) and macroscopic examination. However, 11 patients (42.3%) exhibited MRI-negative microscopic dysplasia in the resected temporal lobe specimens on histological examination.
There were significant correlations between cell genesis and GCD (r=0.43, p=0.03) and between neuronal loss and GCD (r=0.73, p<0.01). Clinical variables, including age at onset, age at surgery, duration of epilepsy, and mean frequency of preoperative seizures, were not correlated with the pathological variables, including neuronal loss, cell genesis, and GCD (Table 3). We dichotomized the results of nestin immunoreactivity into a mild degree of cell genesis (grades 1 and 2) and a moderate-to-severe degree (grades 3 and 4). Moderate-to-severe cell genesis occurred more frequently in patients with a history of FS (p=0.03)(Fig. 2) and in those with frequent generalized seizures (p=0.026, both by chi-square test)(Fig. 2). Neuronal loss and GCD were not significantly correlated with FS or with the frequency of generalized seizures. The presence of temporal microscopic dysplasia was not significantly correlated with the other pathological findings, including neuronal loss, cell genesis, or GCD, or with any clinical variable including the history of FS and frequent generalized seizures.
We investigated the histological changes (neuronal loss, cell genesis, and GCD) in the hippocampus from mTLE patients. Cell genesis, measured as the number of nestin-positive cells in the granule cell layer, was significantly correlated with the severity of GCD, history of childhood FS, and frequent generalized seizures. However, cell genesis was not associated with the extent of neuronal loss, age of seizure onset, duration of epilepsy, or the mean frequency of seizures in the preoperative period. In addition, the presence of microscopic cortical dysplasia in the surgical temporal lobe specimens was not correlated with other pathologic parameters including the degree of neuronal loss, cell genesis, and GCD. These results suggest that newly generated granule cells lead to GCD and are likely to be influenced by FS in childhood and by frequent episodes of generalized seizures.
Granule cell neurons are generated throughout life from a population of continuously dividing progenitor cells that reside in the subgranular zone of the rodent dentate gyrus.29 This also seems to occur in humans, because new neurons, as identified by bromodeoxyuridine staining, are generated from dividing progenitor cells in the dentate gyrus of the adult human brain.30 Prolonged seizure activity markedly increases neurogenesis in the dentate gyrus of adult rats. Pulse-chase bromodeoxyuridine labeling and immunohistochemistry for immature neuronal markers show that many newly generated neurons migrate in chains from the dentate subgranular zone to ectopic locations in the hilus and molecular layer after pilocarpine-induced SE.26 Thus, one hypothesis is that GCD in humans with TLE results from enhanced neurogenesis induced by prolonged seizures. Although our results support this hypothesis, some recent studies have shown contrary results. For example, in a rodent model of epilepsy after kainate injection and in the hippocampi from patients with TLE, GCD does not result from increased neurogenesis, but rather from abnormal migration of mature granule cells along a radial glial scaffold, most likely caused by local reelin deficiency.15,31 Nestin, which is an indicator of cell genesis, is expressed in the neuroglial cells in addition to undifferentiated precursors of neurons.22 Therefore, nestin positivity cannot be attributed entirely to neurogenesis, and this may explain some of the differences between our data and those of previous studies.
We demonstrated increased cell genesis in patients with frequent generalized seizures. Likewise, seizure-induced neurogenesis in the dentate gyrus has been proven in various animal models, such as, pilocarpine-induced SE,20,21,32 kainic acid injection,33 and amygdala kindling.34 Seizure-induced neurogenesis has also been described in adults16 and in children with mTLE.35,36 Although the role of seizure-induced neurogenesis in the pathophysiology of TLE is uncertain, it is possible that newly generated cells contribute to the formation of GCD and ectopic granule cells in the hilus. Seizure-induced cell proliferation and the likelihood of developing spontaneous recurrent seizures following pilocarpine-induced SE are reduced by the antimitotic agent cytosine-b-D-arabinofuranoside in adult rodent models, which suggests that hippocampal cell proliferation plays a proepileptic rather than a compensatory role.20
Experimentally prolonged FS results in late-onset limbic (temporal lobe) epilepsy,37 but the epileptogenic potential of prolonged FS in humans remains unclear. Although retrospective analysis has implicated early-life FS as a risk factor for the development of TLE in humans,38 whether early-life FS actually causes TLE or is simply indicative of another, perhaps genetically determined vulnerability that eventually results in TLE, cannot be determined in correlative clinical studies.37 Moreover, the epileptogenic mechanisms underlying FS remain unknown, but might involve enduring changes at the molecular and functional levels, such as alterations in neurotransmitter receptors or voltage-gated ion channels, and might not involve neuronal loss.39 Although the effects of FS on cell proliferation in the dentate gyrus have been described in animal models,40,41 there is little evidence of this in humans. We found a positive correlation between early-life FS and ongoing cell proliferation in adult human hippocampi taken from TLE patients, but further parallel human and animal studies are needed to demonstrate the role of altered cell proliferation after FS and other epileptogenic mechanisms of FS.
We found a positive correlation between the severity of HS (i.e., neuronal loss) and the presence of GCD, which is in accordance with the results of recent studies5,6,13 and with the original work by Houser.3 Considering that the presence of GCD was not correlated with clinical factors such as duration of epilepsy or frequency of seizures, GCD might be more closely linked to the pathological process of HS rather than being a manifestation of severe temporal lobe seizures.6 We also found no correlation between clinical variables relating to seizure burden and the severity of HS. This is a conflicting area, and most pathological or longitudinal MRI studies have suggested a correlation between the severity of hippocampal neuronal loss and the duration of epilepsy.13 However, few studies have thoroughly determined of the burden of seizures,13 and one limitation to our study is that we did not know the exact total number of generalized and partial seizures. Despite this limitation, we found no evidence that HS occurs as a consequence of recurrent seizures in patients with TLE.
Microscopic cortical dysplasia or microdysgenesis manifests as minor abnormalities on histological examination, even when the MRI or macroscopic examination reveals no abnormalities.42 Microscopic cortical dysplasia may appear in 20-45% of surgical specimens from mTLE patients with HS.43-45 We found microscopic cortical dysplasia in 11 patients (42.3%), a rate similar to that of previous reports, suggesting that it is a relatively commonly associated finding in HS. It has been suggested that this represents a preexisting susceptibility factor that renders the affected brain vulnerable to the development of mTLE after IPI or FS, but the reciprocal relationship remains unknown.43 Recent data show that microscopic cortical dysplasia is not related to clinical parameters such as IPI or FS, or to the histological characteristics of HS;43-45 our results are consistent with these previous findings. Hence, the role of microscopic cortical dysplasia is currently obscure, and future studies are needed to reveal the complexities.
The semiquantitative scoring system that we applied has some limitations. We were unable to quantify real cell counts for neuronal loss and cell genesis, or to measure the real thickness of granule cell layer for GCD, because the tissue sections of the surgical specimens did not precisely correspond. Our results may thus be inconclusive; nevertheless, we think that our results might be suggestive of the neuropathologic features of human specimens.
In conclusion, our study showed that increased cell genesis is correlated with the severity of GCD in the human hippocampal dentate gyrus of medically intractable mTLE patients, supporting the view that newly generated granule cells might lead to GCD. The degree of cell genesis was also related to the history of childhood FS and frequent generalized seizures, but was not significantly associated with the degree of neuronal loss or other clinical variables, such as the age at onset, duration of epilepsy, or the mean frequency of all seizures.
Figures and Tables
Fig. 1
Histopathological results of the semiquantitative scoring system. Nissl staining (for neuronal loss) shows (A) grade 1, 20% of neurons lost, (B) grade 2, 20-50% of neurons lost, and (C) grade 3>50% of neurons lost. Nestin immunostaining (for cell genesis) shows (D) grade 1, few positive cells present (<3 cells/HPF), (E) grade 2, a few positive cells present (3-10 cells/HPF), (F) grade 3, a moderate number of positive cells present (10-100 cells/HPF), and (G) grade 4, a high number of positive cells present (>100 cells/HPF). Prox1 immunostaining (for GCD) shows (H) grade 2, granule cell bodies dispersed into the molecular layer forming an irregular outer border, (I) grade 3, double-layer granule cell bodies organized in two layers, and (J) grade 4, granule cell death prevails. Scale bar=100 µm. GCD: granule cell dispersion.
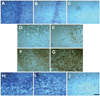
Fig. 2
The distribution of (A) patients with or without a history of FS and (B) with frequent or rare generalized seizures, relative to the degree of nestin positivity (cell genesis). A mild degree of cell genesis was classified as the pathological grading score of nestin of 1 or 2, and a moderate-to-severe degree of cell genesis was classified as grade 3 or 4. Cell genesis was significantly higher in patients with a history of FS (p=0.030) and with frequent generalized seizures (p=0.026). FS: febrile seizures.

Acknowledgements
This study was supported by the Ministry of Health and Welfare (no. A060452), Republic of Korea.
Dr. J-K. Roh was supported by a grant from Seoul National University Hospital, South Korea (no. 2120040140).
References
1. Babb TL, Brown WJ. Engel J, editor. Pathological findings in epilepsy. 1987. New York: Raven Press;511–540.
2. Meencke HJ, Veith G. Luders H, editor. Hippocampal sclerosis in epilepsy. Epilepsy surgery. 1991. New York: Raven Press;705–715.
3. Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res. 1990. 535:195–204.

4. Lurton D, El Bahh B, Sundstrom L, Rougier A. Granule cell dispersion is correlated with early epileptic events in human temporal lobe epilepsy. J Neurol Sci. 1998. 154:133–136.

5. El Bahh B, Lespinet V, Lurton D, Coussemacq M, Le Gal La Salle G, Rougier A. Correlations between granule cell dispersion, mossy fiber sprouting, and hippocampal cell loss in temporal lobe epilepsy. Epilepsia. 1999. 40:1393–1401.

6. Thom M, Sisodiya SM, Beckett A, Martinian L, Lin WR, Harkness W, et al. Cytoarchitectural abnormalities in hippocampal sclerosis. J Neuropathol Exp Neurol. 2002. 61:510–519.

7. Blümcke I, Thom M, Wiestler OD. Ammon's horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol. 2002. 12:199–211.

8. Cavanagh JB, Meyer A. Aetiological aspects of Ammon's horn sclerosis associated with temporal lobe epilepsy. Br Med J. 1956. 2:1403–1407.

10. Babb TL, Brown WJ, Pretorius J, Davenport C, Lieb JP, Crandall PH. Temporal lobe volumetric cell densities in temporal lobe epilepsy. Epilepsia. 1984. 25:729–740.

11. Mathern GW, Adelson PD, Cahan LD, Leite JP. Hippocampal neuron damage in human epilepsy: Meyer's hypothesis revisited. Prog Brain Res. 2002. 135:237–251.

12. Fuerst D, Shah J, Kupsky WJ, Johnson R, Shah A, Hayman-Abello B, et al. Volumetric MRI, pathological, and neuropsychological progression in hippocampal sclerosis. Neurology. 2001. 57:184–188.

13. Thom M, Zhou J, Martinian L, Sisodiya S. Quantitative post-mortem study of the hippocampus in chronic epilepsy: seizures do not inevitably cause neuronal loss. Brain. 2005. 128:1344–1357.

14. Jessberger S, Römer B, Babu H, Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol. 2005. 196:342–351.

15. Fahrner A, Kann G, Flubacher A, Heinrich C, Freiman TM, Zentner J, et al. Granule cell dispersion is not accompanied by enhanced neurogenesis in temporal lobe epilepsy patients. Exp Neurol. 2007. 203:320–332.

16. Thom M, Martinian L, Williams G, Stoeber K, Sisodiya SM. Cell proliferation and granule cell dispersion in human hippocampal sclerosis. J Neuropathol Exp Neurol. 2005. 64:194–201.

17. Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001. 345:311–318.

18. Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius JK. The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain. 1995. 118:105–118.

19. Chu K, Kim M, Jung KH, Jeon D, Lee ST, Kim J, et al. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004. 1023:213–221.

20. Jung KH, Chu K, Kim M, Jeong SW, Song YM, Lee ST, et al. Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur J Neurosci. 2004. 19:3219–3226.

21. Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, et al. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis. 2006. 23:237–246.

22. Kruglyakova EP, Khovryakov AV, Shikhanov NP, Maccann GM 2nd, Vael I, Kruglyakov PP, et al. Nestin-expressing cells in the human hippocampus. Neurosci Behav Physiol. 2005. 35:891–897.

23. Pleasure SJ, Collins AE, Lowenstein DH. Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J Neurosci. 2000. 20:6095–6105.

24. Elliott RC, Khademi S, Pleasure SJ, Parent JM, Lowenstein DH. Differential regulation of basic helix-loop-helix mRNAs in the dentate gyrus following status epilepticus. Neuroscience. 2001. 106:79–88.

25. Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, et al. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002. 129:4249–4260.

26. Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006. 59:81–91.

27. McCloskey DP, Hintz TM, Pierce JP, Scharfman HE. Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci. 2006. 24:2203–2210.

28. Pirttilä TJ, Manninen A, Jutila L, Nissinen J, Kälviäinen R, Vapalahti M, et al. Cystatin C expression is associated with granule cell dispersion in epilepsy. Ann Neurol. 2005. 58:211–223.

29. Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996. 16:2027–2033.

30. Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998. 4:1313–1317.

31. Heinrich C, Nitta N, Flubacher A, Müller M, Fahrner A, Kirsch M, et al. Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. J Neurosci. 2006. 26:4701–4713.

32. Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000. 20:6144–6158.

33. Gray WP, Sundstrom LE. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998. 790:52–59.

34. Parent JM, Janumpalli S, McNamara JO, Lowenstein DH. Increased dentate granule cell neurogenesis following amygdala kindling in the adult rat. Neurosci Lett. 1998. 247:9–12.

35. Blümcke I, Schewe JC, Normann S, Brüstle O, Schramm J, Elger CE, et al. Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus. 2001. 11:311–321.

36. Takei H, Wilfong A, Yoshor D, Armstrong DL, Bhattacharjee MB. Evidence of increased cell proliferation in the hippocampus in children with Ammon's horn sclerosis. Pathol Int. 2007. 57:76–81.

37. Dubé C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006. 129:911–922.

38. Cendes F, Andermann F. Baram TZ, Shinnar S, editors. Do febrile seizures: promote temporal lobe epilepsy? Retrospective studies. 2002. San Diego: Academic Press;78–88.
39. Dube CM, Brewster AL, Richichi C, Zha Q, Baram TZ. Fever, febrile seizures and epilepsy. Trends Neurosci. 2007. 30:490–496.

40. Lemmens EM, Lubbers T, Schijns OE, Beuls EA, Hoogland G. Gender differences in febrile seizure-induced proliferation and survival in the rat dentate gyrus. Epilepsia. 2005. 46:1603–1612.

41. Bender RA, Dubé C, Gonzalez-Vega R, Mina EW, Baram TZ. Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures. Hippocampus. 2003. 13:399–412.

42. Armstrong DD. The neuropathology of temporal lobe epilepsy. J Neuropathol Exp Neurol. 1993. 52:433–443.

43. Kasper BS, Stefan H, Paulus W. Microdysgenesis in mesial temporal lobe epilepsy: a clinicopathological study. Ann Neurol. 2003. 54:501–506.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download