Abstract
Background
It has recently been suggested that diffusion and perfusion MRI can identify subgroups likely to benefit or potentially be harmed by reperfusion therapies.
Case Report
We investigated serial MRI data of two patients with occlusion of the proximal middle cerebral artery (MCA). In both cases, acute multiple cortical infarcts evident on diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI) showed extensive areas of severe perfusion delays, indicating a malignant MRI profile. However, despite the malignant MRI profiles in these cases, no new ischemic lesions or hemorrhage evolved even in the presence of persistent arterial occlusion, and the patients recovered without sequelae.
One of the major concerns in the management of patients with acute ischemic stroke is the identification of individuals who are unlikely to benefit or may even be harmed by recanalization therapy. The Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) trialists recently demonstrated that baseline MRI patterns may be useful for identifying subgroups of patients likely to benefit from reperfusion therapies and also those unlikely to benefit or potentially even be harmed.1 The results of that seminal study are underscored by the relatively large and well-designed nature of the multicenter prospective clinical stroke trial providing a simple but valuable MRI categorization scheme of profiles (mismatch vs. matched vs. small vs. malignant) that are strongly associated with clinical outcomes after reperfusion therapy. They defined a "malignant profile" as a baseline diffusion-weighted imaging (DWI) lesion of 100 mL or more and/or a perfusion-weighted imaging (PWI) lesion of 100 mL or a Tmax delay (time to peak after deconvolution) of 8 seconds or longer, which was associated with a low rate of favorable clinical responses and a high risk of fatal symptomatic intracranial hemorrhage in patients following reperfusion therapies.
We describe two clinical vignettes of acute middle cerebral artery (MCA) occlusion where the malignant MRI profile led to a favorable clinical outcome, emphasizing the critical role of collateral pathophysiology and highlighting potential pitfalls of this malignant MRI pattern derived from time parameters.
A 56-year-old right-handed Korean woman was sitting after having breakfast when she suddenly experienced left arm and leg weakness and dysarthria. On hospital arrival, her NIH Stroke Scale (NIHSS) score was 7 points (arm, 1; leg, 1; dysarthria, 1; face, 2; and neglect, 2). Prior vascular risk factors included hypertension, and she was receiving ongoing treatment with three medications. Other relevant medical history included right thalamic hemorrhage and subarachnoid hemorrhage treated with craniotomy and aneurysm clipping 5 years earlier. Digital subtraction angiography at that time revealed mild atherosclerosis without hemodynamically significant intracranial stenoses.
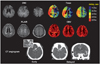
Precontrast and triphasic postcontrast CT scans to obtain sequential images of the early, middle, and late phases obtained using a previously reported method2 showed calcification and total occlusion of the right proximal MCA, with a zone of perfusion abnormalities in the right hemisphere with complete irrigation of the ischemic bed via slow leptomeningeal collaterals evident on middle- and late-phase images (Fig. 1). MRI performed at 4 hours after symptom onset revealed multiple acute small cortical infarcts on DWI. Dynamic susceptibility-enhanced PWI was performed utilizing postprocessing software to generate standard parametric perfusion images, including Tmax maps. Mismatch between perfusion and diffusion data was evident, yet a malignant profile (DWI lesion, 1.5 mL; Tmax >2 sec, 382 mL; Tmax >8 sec, 100 mL) was identified (Fig. 1). A provisional diagnosis of acute thromboembolic infarcts originating from proximal MCA atherosclerosis was established, and the patient was treated with anticoagulation. Her laboratory tests, electrocardiogram, and transesophageal echocardiogram were unremarkable, without suspicion of an alternative stroke etiology.
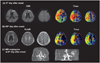
Follow-up MRI was performed on the 8th and 90th days after symptom onset (Fig. 2). Severe perfusion defects were persistently observed but improved on days 8 (Tmax >2 sec, 319 mL; Tmax >8 sec, 81 mL) and 90 (Tmax >2 sec, 233 mL; Tmax >8 sec, 6 mL). Despite the presumed ominous/malignant MRI profile, no new lesions were observed on days 8 (DWI) and 90 [fluid-attenuated inversion recovery (FLAIR) MRI]. Serial time-of-flight MR angiography showed signal loss in the right MCA suggesting persistent arterial occlusion (Fig. 2C) of the proximal MCA. Her initial NIHSS score of 7 points decreased to 2 points on day 8 and to 0 points on day 90 after symptom onset.
A 40-year-old right-handed Korean man awoke early in the morning with left hemiparesis and dysarthria. On hospital arrival, his NIHSS score was 11 (consciousness, 2; arm, 2; leg, 1; face, 2; dysarthria, 1; ataxia, 1; and neglect, 2). Vascular risk factors including hypertension were treated with medications.
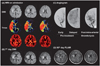
MRI performed 4 hours after symptom onset revealed multiple acute cortical and basal ganglia infarcts on DWI (Fig. 3). A more extensive perfusion abnormality with mismatch was noted throughout the right MCA distribution (DWI lesion, 12.1 mL; Tmax >2 sec, 227 mL; Tmax >8 sec, 123 mL), fulfilling the criteria for a malignant profile. A cerebral angiogram performed immediately after MRI demonstrated occlusive thrombus at the distal M1 segment of the right MCA and good leptomeningeal collateral perfusion. Recanalization with intra-arterial thrombolysis at 6 hours after the time last known well resulted in complete reperfusion [Thrombolysis In Cerebral Infarction (TICI) 3]. Clinical improvement was evident by the NIHSS score decreasing to 4 points at 4 hours post procedure.
Cardiac monitoring and transesophageal echocardiogram showed no evidence of a proximal cardioembolic source or aortic arch atherosclerosis. No new lesions were evident in MRI performed on days 7 and 90. The NIHSS score was 0 on day 90.
These two case examples demonstrate the limitations in using a time-domain parameter such as Tmax to unequivocally define a malignant MRI profile that portends an unfavorable clinical course. Collateral perfusion of the ischemic territory may be characterized by delay, yet dispersion is also an influential feature. PWI data may be analyzed in several ways to yield relative or quantitative values,3 each of which reflect a particular aspect of the collateral perfusion. Time-domain parameters including the mean transit time (MTT; first movement of the signal intensity curve), time to peak, and Tmax have been increasingly used to determine a diffusion-perfusion mismatch.4 Tmax may be a sensitive PWI parameter for detecting an ischemic penumbra and core;5 Tmax thresholds of 6-8 sec were previously found to be optimally predictive of core infarcted tissue at 7 days.5 Several recent and ongoing clinical trials evaluating the effects of reperfusion therapies have based penumbral definitions on the Tmax delay.1,4,6 However, using such time-domain perfusion parameters may have considerable drawbacks in certain scenarios. The patients described in this report had a malignant MRI profile based on the DEFUSE definitions that employ Tmax. In the DEFUSE trial, such "malignant" cases did not attain a favorable clinical outcome after early reperfusion; instead, early reperfusion was associated with fatal intracranial hemorrhage. Our cases did not develop intracranial hemorrhage or any derangement of the blood-brain barrier as measured with T2*-permeability MRI.7 Both cases resulted in optimal clinical outcomes on day 90, including one case with benign oligemia (case 1) and the other case treated with reperfusion therapy (case 2). Other perfusion parameters that more directly reflect the hemodynamic milieu in the ischemic territory suggested that these areas of Tmax delay manifested low perfusion hyperemia or benign oligemia. Such areas of benign oligemia were not necessarily destined for infarction or prone to hemorrhage despite the severe Tmax delay. Prominent dispersion and relative preservation or augmentation of the cerebral blood volume (CBV) sustained the ischemic regions in both cases.
Tmax solely provides an estimate of the delay in bolus arrival time between the arterial input function and a given voxel, without describing the hemodynamic status of the tissue itself.8 Limitations of PWI have previously been described in patients with moyamoya syndrome, which is a model of chronic occlusion;9 long delays in perfusion were observed regardless of the presence or absence of clinical symptoms. The presence of collaterals can introduce both delay and dispersion in the bolus of contrast agent, limiting quantification of PWI data based on normal arterial inflow assumptions.9,10 However, such delay and dispersion features may reflect important vascular pathophysiology such as sustained CBV in downstream regions due to collateral perfusion. Compared to chronic hemodynamic insufficiency, there has been relatively little attention on the role of pretreatment collateral circulation in patients with acute ischemic stroke who are candidates for recanalization therapy.11 Atherosclerotic occlusion in the present cases may have accentuated the role of collateral circulation and the relative importance of dispersion or CBV, beyond the simplistic measure of Tmax delay alone. Inherent delay and dispersion of the collateral perfusion may also cause errors in deconvolution estimates, underestimating cerebral blood flow and overestimating or exaggerating time-domain parameters such as MTT and Tmax.10 This may have been responsible for the severe Tmax delays in both of our cases being falsely interpreted as an ischemic core rather than benign oligemia. Such false interpretation of PWI findings may have obscured the prognosis in case 1 (benign oligemia), and the dramatic clinical benefit of thrombolysis may have been incorrectly overlooked in case 2.
A multiparameter approach has been suggested for defining the PWI abnormalities, combining data from relative cerebral blood flow, regional CBV, and time-domain maps,12 whereas others have advocated simply using time-domain PWI parameters as estimates of tissue at risk because of current limitations of non-time-domain PWI parameters with conventional dynamic susceptibility contrast techniques.6 It has recently been demonstrated that the apparent perfusion lesion size differed markedly depending on which of 10 different PWI postprocessing methods were applied.3 Our present cases suggest that in certain clinical settings of acute ischemic stroke, time-domain PWI parameters should be interpreted with caution, and a non-time-domain PWI parameter may be needed,12 such as in populations with a high prevalence of atherosclerosis (e.g., Asian) or in patients with presumed atherosclerotic stroke and those with prominent collaterals at the time of PWI data acquisition. Further prospective studies should investigate the accuracy of penumbral estimation under these conditions.
Figures and Tables
Fig. 1
MRI and CT angiography findings for patient 1 obtained on admission. DWI: diffusion-weighted imaging, FLAIR: fluid-attenuated inversion recovery, CBV: cerebral blood volume.
References
1. Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006. 60:508–517.

2. Lee KH, Cho SJ, Byun HS, Na DG, Choi NC, Lee SJ, et al. Triphasic perfusion computed tomography in acute middle cerebral artery stroke: a correlation with angiographic findings. Arch Neurol. 2000. 57:990–999.

3. Kane I, Carpenter T, Chappell F, Rivers C, Armitage P, Sandercock P, et al. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke: effect on lesion size, proportion of patients with diffusion/perfusion mismatch, clinical scores, and radiologic outcomes. Stroke. 2007. 38:3158–3164.

4. Davis SM, Donnan GA, Butcher KS, Parsons M. Selection of thrombolytic therapy beyond 3 h using magnetic resonance imaging. Curr Opin Neurol. 2005. 18:47–52.

5. Shih LC, Saver JL, Alger JR, Starkman S, Leary MC, Vinuela F, et al. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke. 2003. 34:1425–1430.

6. Butcher KS, Parsons M, MacGregor L, Barber PA, Chalk J, Bladin C, et al. Refining the perfusion-diffusion mismatch hypothesis. Stroke. 2005. 36:1153–1159.

7. Bang OY, Buck BH, Saver JL, Alger JR, Yoon SR, Starkman S, et al. Prediction of hemorrhagic transformation after recanalization therapy using T2*-permeability magnetic resonance imaging. Ann Neurol. 2007. 62:170–176.

8. Zaharchuk G. Theoretical basis of hemodynamic MR imaging techniques to measure cerebral blood volume, cerebral blood flow, and permeability. AJNR Am J Neuroradiol. 2007. 28:1850–1858.

9. Calamante F, Ganesan V, Kirkham FJ, Jan W, Chong WK, Gadian DG, et al. MR perfusion imaging in Moyamoya Syndrome: potential implications for clinical evaluation of occlusive cerebrovascular disease. Stroke. 2001. 32:2810–2816.
10. Calamante F, Gadian DG, Connelly A. Delay and dispersion effects in dynamic susceptibility contrast MRI: simulations using singular value decomposition. Magn Reson Med. 2000. 44:466–473.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download