Abstract
Background
The main complication of cerebral cavernous angioma is hemorrhage. Ischemic stroke as a complication of cerebral cavernous angioma has rarely been described, and hemorrhage after ischemic Wallenberg's syndrome has not been reported before.
Case Report
A 45-year-old woman presented with perioral numbness, hoarseness, dysphagia, and worsening of her previous sensory symptoms. The patient had been taking aspirin for 3 years after suffering from ischemic Wallenberg's syndrome with left paresthesia as a residual symptom. Brain computed tomography revealed an acute medullary hematoma in the previously infarcted area. Follow-up magnetic resonance imaging revealed a cavernous angioma in the right medulla.
Hemorrhage in the medulla oblongata is a rare form of parenchymal hemorrhage. Medullary hemorrhage has been attributed to hypertension,1 anticoagulant therapy,2 hemangioblastoma,3 and vascular malformation.4-6 Although hemorrhagic transformation after lateral medullary infarction has been reported,7 there has been no report of medullary hemorrhage in the old infarction. We report herein a case of hemorrhage in a previously infarcted area that appeared during oral aspirin treatment for previous ischemic Wallenberg's syndrome in a patient who was subsequently diagnosed as having a cavernous angioma in the right medulla.
An otherwise healthy 45-year-old woman presented with dizziness and dysarthria lasting for 3 days. Neurological examination revealed fragmented Wallenberg's syndrome including hoarseness, hypesthesia on the left hemibody (sparing the face), and gait disturbance. Magnetic resonance imaging (MRI) of the brain demonstrated ischemic infarction of the right lateral medulla (Fig. 1A, B and C), but no evidence of parenchymal hemorrhage, intramural hematoma of the arteries, or leukoaraiosis in the periventricular white matter. Magnetic resonance angiography (MRA) provided no evidence of an underlying vascular malformation. Transcranial Doppler and carotid duplex ultrasonography revealed no evidence of stenosis. Extensive diagnostic studies including transesophageal echocardiography revealed no cardiac or paradoxical source of embolism, and there were no abnormalities on coagulation work-up. Oral aspirin treatment (100 mg/day) was started, and cilnidipine (10 mg/day) was added before discharge because the patient was found to be hypertensive. The neurological deficits slowly improved, but the left paresthesia persisted.
Three years later the patient developed sudden onset of perioral numbness, followed by worsening of the previous sensory symptoms, vomiting, hoarseness, and dysphagia. A physical examination revealed normal vital signs except for blood pressures of 180/100 mmHg. Neurological examination revealed ocular lateropulsion to the left, gaze-evoked counterclockwise torsional and down-beat nystagmus, hypesthesia on the left hemibody including the face, glossopharyngeal paresis, tongue deviation to the right, and marked gait ataxia, but no Horner's syndrome, diplopia, facial weakness, hearing loss, or limb weakness. Computed tomography of the brain revealed an acute medullar hematoma in the previously infarcted area (Fig. 1D). Coagulation panels were within normal limits. Ataxia improved after conservative treatment including rehabilitation, and the patient was discharged on hospital day 35. A follow-up MRI performed approximately 3 months after the initial hemorrhage produced findings suggestive of cavernous angioma in the right dorsomedial medulla (Fig. 1E-H).
Even though pathological confirmation was not achievable, this case probably represented cerebral cavernous angioma, and not hemorrhagic metastasis, which was another consideration. Differentiating between cavernous angioma and hemorrhagic tumor is not clear-cut. However, benign hemorrhage is characterized by a complete hemosiderin ring and lack of surrounding edema.8 Other diagnostic possibilities include hypertensive hemorrhage in association with cerebral microbleeds, hemorrhagic infarction, and arteriovenous malformation. Cerebral microbleeds, which are tiny extravasations of blood from lipohyalinized small penetrating cerebral arterioles, may be of importance because these lesions are associated with spontaneous hemorrhage.9 We do not know whether our patient had these lesions in the medulla prior to the ischemic episode. Although it has been demonstrated that hypertension is a risk factor for cerebral microbleeds,10 the patient had no hypertensive changes such as retinopathy, left ventricular hypertrophy, or small-vessel changes on MRI. The patient did have high blood pressure during the hemorrhage, but her blood pressure had since been controlled adequately.
This patient developed hemorrhage 3 years after suffering from ischemic Wallenberg's syndrome. The possibility of hemorrhagic infarction by reperfusion injury seems quite unlikely given the time interval between the infarction and the hemorrhage. Although there was no evidence of cavernous angioma in initial MRI, we presume that the ischemia was caused by the cavernous angioma based on the initial ischemic lesion of this patient being located more medially than is typical for ischemic Wallenberg syndrome caused by cardioembolic, large-artery, or small-vessel diseases, and the hemorrhage developing in the same area in which the ischemia had occurred previously. In patients suspected to have an arteriovenous malformation, MRA or conventional angiography would be helpful in detecting lesions and demonstrating blood flow within those lesions. MRA revealed no evidence of arteriovascular malformation in this patient.
Cerebral cavernous angiomas reportedly account for 5-13% of all vascular malformations. The main complication of cerebral cavernous angioma is hemorrhage, but ischemic stroke has been described in the form of Wallenberg's syndrome as the presenting symptom of cavernous angioma.11 Cavernous angiomas present a dynamic balance between intracavernous bleeding and thrombosis. An increase in the intracavernous pressure may cause bleeding, and a reduced blood flow may be responsible for thrombosis.11 Disturbance of this balance may lead to either ischemia or hemorrhage. Cantu et al.12 reported that the nonlobar location of cavernous angiomas confers a higher risk of hemorrhage, and that the hemorrhagic rate was 2.33% per patient per year for brainstem angiomas, but 1.22% per patient per year for lobar angiomas. Kattapong et al.8 demonstrated several characteristic patterns of cavernous malformation on MRI. The largest lesions showed a reticulated pattern, the smallest lesions had a primarily hemosiderin pattern, and medium-sized lesions usually exhibited a target pattern, as in the present case.
Our patient received no anticoagulant therapy, and extensive study demonstrated no hemorrhagic diathesis, but she had been taking aspirin since the initial ischemic episode that had occurred 3 years previously. Prior use of antithrombotic drugs is known to be a risk factor for hemorrhage after ischemic stroke.13,14 Although aspirin is widely used for the prevention of stroke, and the benefits outweigh its hemorrhagic risks, prolonged use of aspirin is associated with an increased risk of hemorrhage.15 Hemostatic mechanisms are impaired in patients treated with antithrombotic agents, and a small leakage may result in a large hematoma. Although the medulla is not a typical site for microbleeds, aspirin-associated hemorrhages have been described in association with cerebral microbleeds.
We presume that cerebral cavernous angioma was responsible for both the ischemia and the hemorrhage in the present case, and we also cautiously speculate that aspirin contributed to the development of a hemorrhage in the previously infarcted area by rendering the brain prone to bleeding. This case may represent an unusual complication of cerebral cavernous angioma, showing delayed hemorrhage after ischemic Wallenberg's syndrome.
Figures and Tables
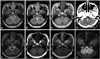 | Fig. 1Brain images of the patient. A-C: Transverse sections of T2-weighted, fluid-attenuated inversion recovery, and T1-weighted MR images taken when the patient had Wallenberg's syndrome 3 years ago show an acute ischemic infarction of the dorsal medulla. D: Brain CT shows hemorrhage in the previously infarcted area. E-H: Follow up MRI including T2-weighted, T1-weighted, after gadolinium enhancement, and a gradient-echo T2-weighted images show target appearance lesion with central high signal and surrounding low signal of hemosiderin deposition which is more conspicuous on gradient-echo image, one of the patterns of cavernous angioma. |
References
1. Kwon HM, Park JM, Lee JY, Yoon BW. Primary medullary hemorrhage with hypertension. J Clin Neurol. 2005. 1:177–179.
2. Mastaglia FL, Edis B, Kakulas BA. Medullary haemorrhage: a report of two cases. J Neurol Neurosurg Psychiatry. 1969. 32:221–225.

3. Ryang YM, Oertel MF, Thron A, Gilsbach J, Rohde V. Rare intramedullary hemorrhage of a brainstem hemangioblastoma. Zentralbl Neurochir. 2007. 68:29–33.

4. Kempe LG. Surgical removal of an intramedullary haematoma simulating Wallenberg's syndrome. J Neurol Neurosurg Psychiatry. 1964. 27:78–80.

5. Cohen HC, Tucker WS, Humphreys RP, Perrin RJ. Angiographically cryptic histologically verified cerebrovascular malformations. Neurosurgery. 1982. 10:704–714.

6. Chaudhuri A, Shah PU. Spontaneous medullary haemorrhage due to occult vascular malformations. J Assoc Physicians India. 1996. 44:424–426.
7. Jung HH, Baumgartner RW, Hess K. Symptomatic secondary hemorrhagic transformation of ischemic Wallenberg's syndrome. J Neurol. 2000. 247:463–464.

8. Kattapong VJ, Hart BL, Davis LE. Familial cerebral cavernous angiomas: clinical and radiologic studies. Neurology. 1995. 45:492–497.
9. Lee SH, Bae HJ, Kwon SJ, Kim H, Kim YH, Yoon BW, et al. Cerebral microbleeds are regionally associated with intracerebral hemorrhage. Neurology. 2004. 62:72–76.

10. Tanaka A, Ueno Y, Nakayama Y, Takano K, Takebayashi S. Small chronic hemorrhages and ischemic lesions in association with spontaneous intracerebral hematomas. Stroke. 1999. 30:1637–1642.

11. Cardona-Portela P, Escrig-Avellaneda A, Rubio-Borrego F. [Wallenberg's syndrome as the presenting symptom of a cavernous angioma]. Rev Neurol. 2004. 39:837–840.
12. Cantu C, Murillo-Bonilla L, Arauz A, Higuera J, Padilla J, Barinagarrementeria F. Predictive factors for intracerebral hemorrhage in patients with cavernous angiomas. Neurol Res. 2005. 27:314–318.

14. Nighoghossian N, Hermier M, Adeleine P, Blanc-Lasserre K, Derex L, Honnorat J, et al. Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke: a gradient-echo T2*-weighted brain MRI study. Stroke. 2002. 33:735–742.

15. Wong KS, Mok V, Lam WW, Kay R, Tang A, Chan YL, et al. Aspirin-associated intracerebral hemorrhage: clinical and radiologic features. Neurology. 2000. 54:2298–2301.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download