Abstract
Deep brain stimulation (DBS) surgery has been performed in over 75,000 people worldwide, and has been shown to be an effective treatment for Parkinson's disease, tremor, dystonia, epilepsy, depression, Tourette's syndrome, and obsessive compulsive disorder. We review current and emerging evidence for the role of DBS in the management of a range of neurological and psychiatric conditions, and discuss the technical and practical aspects of performing DBS surgery. In the future, evolution of DBS technology may depend on several key areas, including better scientific understanding of its underlying mechanism of action, advances in high-spatial resolution imaging and development of novel electrophysiological and neurotransmitter microsensor systems. Such developments could form the basis of an intelligent closed-loop DBS system with feedback-guided neuromodulation to optimize both electrode placement and therapeutic efficacy.
As a result of both improved technology and understanding of neurological diseases, there has been a significant increase in the application of restorative functional neurosurgical techniques to treat neuropsychiatric disorders. Among these newer surgical therapies, electrical stimulation of specific subcortical brain nuclei, known commonly as deep brain stimulation (DBS), has become an increasingly popular alternative to pharmacological treatment alone. At this time, more than 75,000 people have been successfully implanted with DBS devices worldwide, and this number is expected to expand rapidly. DBS is now approved by the US Food and Drug Administration (FDA) and is in routine clinical use for treatment of, Parkinson's disease (PD),1 essential tremor (ET),2-4 dystonia5 and obsessive compulsive disorder (OCD).6,7 Furthermore, there is growing evidence for the use of DBS in the treatment of disorders such as depression,8-10 epilepsy,11-13 Tourette syndrome (TS),14 and chronic pain.15,16 Here, we review current and emerging evidence for the role of DBS in the management of a range of neurological and psychiatric conditions, discuss the potential mechanism of action, and explore the future application of this exciting technology.
PD is associated with a severe loss in function of dopaminergic neuronal cells within the substantia nigra pars compacta which project to the striatum, a major component of the basal ganglia. Progressive degeneration of these nigrostriatal projections leads to motor symptoms such as tremor, rigidity, bradykinesia, and postural instability. While PD patients have benefited significantly from the development of new pharmacological treatments used in combination with the traditional drug levodopa, many of these therapies have been either only partially effective or poorly-tolerated over the long course of the disease.17,18 In addition, these pharmacological therapies are also associated with serious and debilitating motor complications, such as dyskinesias.19 However, significant advances in stereotactic and functional neurosurgical techniques over the last 15 years in response to these shortcomings have led to new strategies in the treatment of advanced PD using electrical stimulation.20-22
Although the therapeutic efficacy of DBS in PD has been well-established,23,24 results from the multicenter, open-label PD SURG trial in the UK have shown that DBS plus medical therapy improves patient self-reported quality of life significantly more than best medical therapy alone.25 Importantly, these findings were accompanied by clinically meaningful differences on the Unified Parkinson's Disease Rating Scale (UPDRS), including substantially improved patient ratings of the frequency and severity of dyskinesias and "off" periods.
Today, the most common target for DBS in PD is the subthalamic nucleus (STN) as this ameliorates the cardinal symptoms of bradykinesia, rigidity and tremor.24,26,27 In cases of PD where the patient's main complaints do not involve bradykinesia or rigidity, other anatomical targets for DBS may be appropriate. For example, DBS of the ventralis intermedius (Vim) thalamus, which is common for treating ET, is also an effective target for patients with tremor-dominant PD.28-32 As mentioned, studies have also demonstrated significant overall improvements in PD patients treated with DBS of the globus pallidus interna (GPi).33-42 Although improvements in both gait and posture had been shown,37,43 the main utility of GPi DBS was seen to be in the reduction of dyskinesias often seen with long-term levodopa treatment.36-42,44 However, the results of a recent multicenter, randomized, blinded trial showed that both GPi and STN DBS result in similar improvements in part III (motor subscale) of the UPDRS at 24 months.45 Patients who underwent STN DBS subsequently required lower doses of dopaminergic agents, and the level of depression (as measured by the Beck Depression Inventory II) worsened slightly after STN stimulation but improved slightly after GPi stimulation, although the reported differences are unlikely to be clinically important. Recently, interest has also grown in low frequency stimulation of the pedunculopontine nucleus for gait disorders/freezing and postural instability in PD, with two small double-blinded studies showing a reduction in falls at 1-year.46,47
ET is characterized by rhythmic, involuntary movements commonly affecting the arms, head and voice.48 It is by far the commonest movement disorder, with a prevalence of up to 5.5% in those 65 and over.49 Early promising results for DBS in the treatment of ET50 were replicated and eventually led to a direct comparison study of thalamotomy and DBS of the Vim thalamus for treatment of tremors, including ET.51 In this study, Schuurman et al.51 reported a significant improvement of tremor in both treatment groups, but a greater reduction in the DBS group, who also experienced fewer side effects and a better functional outcome. The marked improvement seen with DBS of the Vim thalamus for upper limb tremor also appears to be maintained during long-term follow-up.52
Dystonia refers to a broad group of conditions with differing aetiologies, which involve abnormal muscle spasm and posturing. In general, they may be primary, associated with a genetic predisposition such as the torsion dystonia 1 (DYT1) gene, or secondary to other causes such as metabolic disorders, drug intake (e.g., tardive dyskinesia), or brain injury (e.g., stroke).55 Dystonias are also classified as generalized, segmental (affecting two adjacent body parts) or focal (e.g., cervical dystonia/spasmodic torticollis, blepharospasm, and writer's cramp) according to the pattern of involvement.
Most evidence for DBS in dystonia highlights the effectiveness of targeting the GPi in primary generalised dystonia56,57 and cervical dystonia. There is also increasing evidence for bilateral GPi DBS in patients with tardive dyskinesia,58-60 a condition which is notoriously difficult to treat medically. Secondary dystonias tend to benefit less from DBS of the GPi, and this may be a consequence of the wide range of aetiologies and brain areas potentially involved.
As highlighted by Kuhn et al.61 the idea of using DBS to treat psychiatric disorders stems from several observations and developments: 1) some PD patients treated with DBS developed psychiatric adverse effects or experienced improvement of a co-morbid psychiatric disorder; 2) lesion procedures for intractable psychiatric disorders have yielded positive results, but were avoided because of their severe adverse effects; 3) identification of potential anatomical targets for DBS in psychiatric disorders has improved due to a growing body of functional neuroimaging studies. The role of neuromodulation in the treatment of refractory psychiatric disorders has been extensively reviewed, including by our own group62 and more recently by others.63 Here, we will only provide an overview of current thinking about the role of DBS in treatment-resistant cases of depression, OCD and TS.
In the United States, major depression may affect almost 1 in 5 people64 and up to 20% of patients fail to respond to first-line pharmacological interventions.65 Early studies have implicated the subgenual cingulate cortex (Cg25) in acute sadness and antidepressant effects,66,67 and a decrease in Cg25 activity has been associated with immediate clinical response to a number of antidepressant treatments including serotonin reuptake inhibitors,68 electroconvulsive therapy,69 transcranial magnetic stimulation,70 and ablative surgery.71 As such, early research by Mayberg et al.9 focused on bilateral Cg25 white matter (Cg25WM) DBS for treatment-resistant depression and resulted in the publication of a preliminary report of six patients9 and a final report of twenty patients.72 In the final report, after 6 months 12/20 patients had a reduction of at least 50% in the 17-item Hamilton Rating Scale for Depression (HRSD-17) score and 7 patients met criteria for remission (HRSD-17 ≤7). Positron emission tomography (PET) of cerebral blood flow (CBF) in the pilot study (5/6 patients) showed increased Cg25 CBF and decreased CBF in lateral prefrontal and anterior cingulate cortices relative to controls, which reversed with stimulation. Of the additional 14 patients in the final study, 18-Fluoro-deoxyglucose-PET (FDG-PET) results from 8 patients responsive to DBS similarly showed widespread changes in cortical and limbic metabolic activity, including increased activity in lateral prefrontal cortex and Cg25WM, but a reduction in Cg25 grey matter.
Other potential targets relate to areas which the Cg25 sends and receives its projections: the nucleus accumbens (NAcc) [also referred to as the ventral striatum (VS)] and limbic cortical loop.73 The NAcc forms an interface between emotional, limbic and motor neuronal circuits and plays a vital processing role in the experience of reward and hedonistic stimuli.61 In a study by Schlaepfer et al.10 3 patients with treatment resistant depression underwent bilateral NAcc DBS. Within one week of stimulation onset the HRSD-24 decreased by an average of 42% and demonstrated that stimulation was inversely correlated with depression. Interestingly, while FDG-PET after 1 week of NAcc DBS only showed activation of dorsal prefrontal and cingulate cortices (with no change in Cg25 activity), subsequently published expanded data for the first 10 patients74 showed decreased metabolism in both the Cg25 and prefrontal regions on FDG-PET taken at 6 months. In this study, 5 patients reached 50% reduction in HRSD-28 at 1 year and anxiety was reduced in the whole group, but to a greater degree in the responders.
Previously, studies targeting the ventral anterior internal capsule (VC)/VS treatment-resistant OCD patients have also shown improvements in depressive symptoms.7,75-80 As such, Malone et al.81 attempted bilateral VC/VS DBS in 15 patients with treatment-resistant depression. They found that the proportion of patients with at least 50% reduction in HRSD-24 was 47% at 3 months, 40% at 6 months and 53% at last follow up, while remission rates with HRSD-24 were 20% at 6 months and 40% at last follow up.81
OCD is characterized by recurring, anxiety-provoking thoughts (obsessions) and repetitive behaviors (compulsions), and affects approximately 2% to 3% of the general population.62,82 The FDA granted DBS a humanitarian device exemption for medically refractory OCD in 2009 on the basis of several clinical trials showing positive findings. As such, the main targets currently used for DBS in OCD are the VC/VS,79 the shell region of the NAcc, and the STN.
Early studies showed that bilateral VC/VS DBS had a beneficial impact on OCD symptoms, but required unusually high stimulation amplitudes (5.0-10.5 V), suggesting that perhaps the therapeutic target was adjacent to, but not at the electrode site. Taken together, the high stimulation amplitudes and electrode site (the ventral edge of the internal capsule, where it abuts the NAcc) has prompted suggestions that functional blocking of NAcc activity may be underlying the symptomatic improvement.83 Indeed, a study of unilateral right NAcc DBS in 4 patients with refractory OCD showed near complete recovery in 3 out of 4 patients using lower stimulation amplitudes (2.0-6.5V).83 Recently, in a double-blind sham-controlled crossover study of unilateral right NAcc DBS in 10 patients with treatment-resistant OCD, Huff et al.84 reported a full response [>35% reduction in Yale-Brown Obsessive Compulsive Scale (Y-BOCS)85] in one patient and partial responses (25-35% reduction in Y-BOCS85) in 4 other patients at 1 year. Despite a different target and only using unilateral stimulation, these results are broadly consistent with earlier studies7,78,86 where about 50-60% of patients acquired a ≥25% reduction in Y-BOCS score within one year of DBS. While the majority of patients in these earlier studies had full responses, Huff et al.84 suggest that this may be due to differences in stimulation protocol, anatomical target, use of bilateral stimulation, and effectiveness of blinding.
In patients with OCD who underwent STN DBS for their PD symptoms, studies have also reported unintentional psychiatric benefits.87,88 In view of this, Mallet et al.89 performed a randomized double-blind crossover study of bilateral anteromedial STN DBS for treatment-resistant OCD, with two parallel groups of 8 patients undergoing two sequential 3-month blinded phases ("stimulation on" followed by "stimulation off" vs. "stimulation off" followed by "stimulation on"). This study found a significant reduction in the Y-BOCS score after the on-stimulation phase compared to after the sham-stimulation, but urged caution after observing 15 serious adverse effects in 11 patients (including one intracerebral haemorrhage and 2 infections).
TS is characterized by repetitive, stereotyped, involuntary movements and vocalizations called tics.62 These can be simple motor tics (e.g., eye blinking, facial grimacing, shoulder shrugging, repetitive throat-clearing), complex tics (combinations of movements) and vocal tics such as coprolalia (uttering swear words) or echolalia (repeating the words or phrases of others). The early symptoms of TS are generally noticed in childhood, with an average age of onset of 7 to 10 years.90 Although in most cases the disorder is self-limiting or amenable to treatment, some patients have an intractable form which may be helped by DBS.91
In 1999, DBS was trialed as a new approach for intractable TS.92 Maciunas et al.14 conducted the first prospective double-blind crossover trial of DBS in five adults with TS, utilizing bilateral stimulation of the centromedian-parafascicular thalamic nuclei (CM-Pf). In the initial 4-week blinded phase patients spent each week in one of 4 electrode states [no stimulation; unilateral (right-sided) stimulation only; unilateral (left-sided) stimulation only; bilateral stimulation], followed by 3 months in the bilateral stimulation state. During the initial blinded phase, a >50% reduction in tics was seen in 3/5 patients during the bilateral stimulation week (versus no stimulation) as measured by modified Rush Video-Based Rating Scale and Yale Global Tic Severity Scale (YGTSS), which fell to 2/5 patients after 3 months of open stimulation. In another double-blind, randomized crossover trial 3 patients with medically refractory TS were implanted with DBS electrodes in the CM-Pf and GPi bilaterally (i.e., 4 electrodes per patient).93 In the 8-month blinded phase, patients experienced 2 months each of CM-Pf, GPi, combined CM-Pf/GPi, and sham stimulation. Interestingly, the reduction in the YGTSS was greater during bilateral GPi stimulation alone (about 78%) compared to bilateral CM-Pf stimulation (about 45%), and even compared to simultaneous CM-Pf/GPi stimulation (about 60%).
In another uncontrolled study, 18 cases of TS underwent DBS placed bilaterally in the CM-Pf and ventralis oralis complex of the thalamus.94 Although follow up ranged from 3-18 months, the average YGTSS fell by about 70% after 12 months. The same group has recently published the 2-year outcomes for 15 of the original 18 patients, which showed a sustained reduction in tic severity (about 53%), as well as improvement in obsessive-compulsive symptoms, anxiety symptoms, depressive symptoms, and subjective quality of life.95
Approximately 35% of epileptic patients experience refractory disease unresponsive to antiepileptic drugs.96 While some may benefit from resective surgery, patients who have seizures arising from eloquent cortex, or which are multifocal, bilateral, or generalized, are not candidates for resective surgery and can be considered for alternative therapy.97 In this context, DBS aims to reduce seizures by modulating subcortical systems which can remotely control seizure generators. Indeed, previous studies have looked at DBS applied to a number of subcortical targets, including the cerebellum, various thalamic nuclei, and several structures of the basal ganglia system (reviewed by Kahane and Depaulis97). However, the recent publication of the results from the multicenter, double-blind, randomized SANTE trial of bilateral DBS of the anterior thalamic nuclei in patients with treatment-resistant partial and secondarily generalized epilepsy represents a significant step. This study showed a 56% reduction in mean seizure frequency at 2 years (n=102), with 54% of patients having at least 50% reduction in seizure frequency.98 While complication rates were modest, 2 participants had transient stimulation-related seizures and during the initial 3 month blinded phase participants in the sti-mulated group were more likely to report depression and memory problems as adverse effects.
DBS for chronic pain has been used since the 1950s, when the caudate and thalamus were targeted in patients with chronic pain. However, the lack of conclusive results from multicentre trials99 undertaken thus far has meant that DBS for pain is performed on an off label basis. In a meta-analysis of 7 studies published from 1977-1997, which included 424 patients undergoing DBS to the PAG/PVG, internal capsule, and thalamus, Bittar et al.16 found a long term success rate of 79% for PAG/PVG compared to only 58% for sensory thalamic stimulation, but 87% when PAG/PVG was combined with sensory thalamic/internal capsule stimulation. Interestingly, this is in contrast to findings of a recent study of 21 patients receiving either ventrocaudalis thalamic nucleus (Vc) or combined Vc and PAG/PVG DBS, where of the 5 patients who experienced long-term pain relief after DBS, 4 had received Vc only DBS.100 The analysis by Bittar et al. also found that patients with failed back surgery syndrome (FBSS) had better outcomes than those with post-stroke pain, phantom limb pain or peripheral neuropathic pain, and that nociceptive pain responded to DBS better than neuropathic pain. More recently, in a study of 56 patients with various neuropathic and mixed nociceptive/neuropathic pain syndromes undergoing DBS of the PVG/PAG and either VPL or VPM, the best results were again seen in patients with FBSS, while poorer outcomes were seen for dysesthesia dolorosa, phantom limb pain and central pain syndromes (spinal cord injury and post-stroke pain).15 Interestingly, smaller studies by a group in Oxford, UK have had significantly better outcomes for phantom limb pain,101 neuropathic pain102 and central pain. Indeed, in a trial of 15 patients with post-stroke pain who underwent DBS of the PVG and VPL, it was initially successful and devices internalized in 12 patients exhibited a nearly 50% reduction in visual analogue pain score over a follow-up period of 27 months.103
The role of DBS in treating neuropathic and other facial pain syndromes has also been studied. Franzini et al.104 targeted the ipsilateral posterior hypothalamus for DBS and reported long-term pain relief in 5 patients with treatment-resistant cluster headache (CH). Subsequently, Schoenen et al.105 studied ipsilateral ventroposterior hypothalamic DBS in 6 patients with intractable CH. Surgery was successfully completed in 4 patients and, of these, 2 were pain-free and 1 had <3 attacks per month, but another only had transient remissions. Long-term results of continuous posterior inferior hypothalamic stimulation in 16 chronic drug-refractory patients with CH showed that 13 patients were persistently pain-free or almost pain-free, and the other 3 are improved at a mean follow-up of 23 months.106 More recently, Green et al.107 studied DBS of the PAG/PVG and/or VPM in 7 patients with a range of neuropathic cephalgias of various aetiologies and demonstrated >50% improvement in their pain scores.
All approaches to DBS surgery broadly combine stereotactic technique with detailed image guidance. Commonly, a stereotactic head frame is placed on the patient under local anesthesia and magnetic resonance imaging (MRI) is performed to identify the anterior commissure, posterior commissure, and the mid-commissural point. Well-established Cartesian (x, y, and z) target coordinates, relative to the mid-commissural point, are used for planning electrode placement. Stereotactic target coordinates are discerned from computer software that merges the MRI of the patient's brain with a brain atlas. At our institution, we routinely utilize gadolinium contrast in our pre-operative MRI with a head frame to visualize the location of blood vessels which, in addition to the ventricles, can then be avoided in the planned electrode trajectory.
Once imaging has been completed and a safe trajectory established, the patient is returned to the operating room where, under sterile conditions and local anesthesia, surgery commences. One or more burr holes are placed in the skull at the predetermined entry points. During surgery microelectrode unit recordings are used to verify a trajectory using region-specific neural activity as functional landmarks.108,109 If suitable results are not obtained, another tract is chosen, and the recording procedure is repeated. Once a trajectory is verified, the microelectrode is withdrawn and the stimulating electrode implanted and test stimulation conducted in the awake patient using a temporary external stimulator. This enables the patient to give verbal feedback confirming the absence of any unwanted side effects from stimulation (e.g., paraesthesias suggesting current spread to the somatosensory thalamus) and provides the surgeon with an opportunity to relocate the electrode if side effects do occur. Confirmation of final electrode position is usually performed first with intraoperative fluoroscopy then postoperative MRI or computed tomography. Once complete and trial stimulation is deemed successful, the implanted stimulating electrode is secured to the skull and connected to a pulse generator that is subsequently implanted subcutaneously, inferior to the clavicle.
As such, DBS surgical procedures can be quite lengthy, requiring the awake patient to undergo many difficult hours of electrophysiological recordings that is necessary to confirm the anatomy scanned by MRI. With each pass of the recording microelectrode there is an increased risk of intracranial hemorrhage.110 Even with this extensive and sometimes precarious implantation procedure, stimulating electrodes may be misplaced, an event associated with side effects such as depression111,112 and even suicidal ideation.112 The state of DBS technology, the surgical procedures, and post-surgical imaging have remained largely static since their inception and remain arduous and time consuming for both patient and practitioner. As such, obtaining optimal lead placement with minimal lead penetrations is of paramount importance and the availability of more refined anatomical information to enable more precise placement of DBS electrodes, together with utilization of real-time electrophysiological and neurotransmitter measurements during surgery, may address many of these critical issues. However, it is clear that the framework for the development of the next generation of DBS devices and surgical approaches is dependent on our understanding of its mechanism of action.
Despite the bourgeoning application of DBS in clinical practice, an understanding of the mechanism(s) underlying its clinical effect is incomplete. The similar effectiveness of STN DBS and ablative surgery targeting the STN (subthalamotomy) initially led to the idea that DBS acted to silence pathologically hyperactive neurons113-116 and this was supported by electrophysiological studies.117,118 Paradoxically, more recent studies have reported the activation of STN output nuclei during DBS.119 However, mathematical modeling has reconciled these two finding by suggesting that, because of dissimilar excitability of neural elements, soma inhibition and axonal activation are both expected at the DBS electrode site.120,121 This axonal activation hypothesis, which has now come to dominate current thinking122-125 proposes that DBS evokes changes in neural activity and neurochemical transmission in interconnected structures within the basal ganglia complex that ultimately underlie clinical benefit. Unfortunately, our present understanding of these distal effects of STN DBS remains far from complete, in large part because of the technical difficulties in combining available modalities for the global assessment of neural activity with those for the detection of specific neurochemicals.
Consistent with the axonal activation hypothesis, electrophysiological recordings during STN DBS have shown increased activity in STN target neurons in the GPi and globus pallidus externa (GPe)126-128 and the substantia nigra pars reticulata and pars compacta (SNr and SNc, respectively).129-131 While definitive, the downside of the electrophysiological approach is that targets must be selected a priori and few targets can be evaluated concurrently. In contrast, brain imaging techniques are ideal candidates for simultaneous global assessment of neural activity during STN DBS.
Several clinical studies utilizing PET, H215O-PET, and FDG-PET support the axonal activation hypothesis of STN DBS.132 PET and H215O-PET record changes in regional cerebral blood flow (rCBF),133,134 while FDG-PET measures regional cerebral glucose metabolism.135 Both rCBF and metabolic activity are considered to reflect altered local neuronal activity or altered input into the region of measurement.136 In a total of 70 PD patients using PET133,137 and H215O-PET132 in the resting state, studies have found that with clinically effective STN DBS there are similar increases in activity in the globus pallidus, thalamus, and SN and decreases in activity in the pre-motor and supplementary motor areas, including the primary motor cortex. Zhao et al.138 using FDG-PET in seven PD patients also found similar increases in activity in basal ganglia structures, including caudate nucleus and putamen (striatum). Taken together, these PET results suggest that the net effect of STN DBS is to increase the activity of STN output, supporting the axonal activation hypothesis.
Clinical studies utilizing functional MRI (fMRI) have also supported the axonal activation hypothesis of STN DBS. The fMRI brain imaging protocol measures blood-oxygenation-level dependent (BOLD) contrast139 that provides in vivo real-time anatomic maps of blood oxygenation in the brain under normal physiological conditions.140,141 In the first attempt to utilize 1.5 Tesla (1.5 T) fMRI in four PD patients during STN DBS, Jech et al.142 showed BOLD signal activation in structures in the basal ganglia complex such as the globus pallidus, thalamus, SN, and cortical structures that included premotor cortex and dorsolateral prefrontal cortex. In a more recent fMRI study examining the effects of STN DBS, Philips et al.143 implanted five PD patients with bilateral DBS electrodes. These investigators reported that BOLD signal activation was seen in the ipsilateral basal ganglia, typically in the caudate nucleus, putamen and GP in all subjects and ipsilateral thalamus in six of the electrodes tested. In another fMRI study of one subject the electrode on the left was within the ventral STN, whereas the right electrode was in the dorsal STN.144 The left STN DBS primarily showed increases in premotor and motor cortex, thalamus, putamen, and cerebellum, as well as decreases in sensorimotor/supplementary motor cortex while the right DBS showed similar but less extensive change in motor regions and unique increases in prefrontal cortex, anterior cingulate, thalamus, caudate nucleus, and brainstem. Where STN DBS effects were specifically examined in PET and fMRI studies, they clearly demonstrate that increases in basal ganglia network activity (including in the striatum) are consistent with the axonal activation hypothesis, but the question of which neurotransmitter systems are responsible remains unresolved.
The cardinal symptoms of PD (akinesia, rigidity and tremor) are associated with severe nigrostriatal dopaminergic denervation145 and levodopa, a biochemical precursor to dopamine and the mainstay of PD treatment, is thought to act by increasing endogenous dopamine synthesis and release.146-148 Bilateral STN DBS reverses the cardinal motor symptoms in PD patients,26,44 and decreases or eliminates the need for levodopa.149,150 However, the hypothesis that DBS of the STN contributes to symptom relief in PD by activation of surviving nigrostriatal dopaminergic neurons, resulting in dopamine release and resumption of target cell control in the striatum, is still controversial. Indeed, it is entirely possible that STN DBS could be altering neuronal circuits downstream of striatal dopamine release to provide therapeutic benefit.
Most basic (animal) studies using in vivo microdialysis, which physically removes analyte from brain extracellular fluid for ex vivo analysis, do not report an increase in striatal dopamine release during high frequency stimulation (HFS) of the STN in intact rats or the 6-hydroxydopamine (6-OHDA)-lesioned rat model of PD.151-153 Additionally, Windels et al.154,155 have shown in rats that STN DBS significantly increased glutamate and GABA release in the GPe and SNr, respectively. However, the relatively large size of microdialysis probes have been shown to disrupt tissue in the immediate vicinity of the probe resulting in underestimations of extracellular dopamine levels compared to alternative measurement techniques that utilize chemical microsensors.156-158 As such, approaches other than microdialysis will be necessary to assess striatal dopamine release during STN DBS. Indeed, chemical microsensors, which offer a smaller probe (5-10 µm versus 200-400 µm diameter for microdialysis probes), have shown dopamine release in the striatum evoked by STN DBS in the intact and 6-OHDA rat model159-161 and in an intact large animal (pig) model.162 These latter findings are important on several levels. For example, striatal dopamine release during STN DBS has been difficult to establish with microdialysis,151-153,163 with one exception,164 a result that underscores the need for application of much smaller microsensors in neurochemical assessments of the effects of STN DBS on distal neurotransmission.
Several in vivo PET studies have also failed to demonstrate significant displacement of the dopamine receptor ligand [11C] raclopride despite significant improvements in motor performance following STN DBS,165-168 potentially suggesting that terminal dopamine release does not underlie its anti-Parkinsonian effects. However, PET scanning with raclopride has relatively poor temporal resolution and requires an increase of greater than 90% of baseline measures in order to detect a change in dopamine efflux.166,169 Additionally, it has been suggested that adaptive changes in dopamine receptor populations (e.g., D2 receptor internalization and/or recycling) occurring over long-term STN stimulation may interfere with PET quantification of dopamine release in these patients.170 However, the fact that DBS of the STN is most effective in PD patients who respond well to levodopa171 and contraindicated for those who do not,172 suggests that effective DBS requires endogenous dopamine production. Additionally, the observations that DBS elicits dyskinesias that resemble those seen with levodopa excess26 and that, like levodopa, it contributes to impulsivity (a behavior thought to be dopamine-mediated)173 are also consistent with activation of surviving dopaminergic neurons by DBS. Thus, whether STN DBS improves PD symptoms via the release of dopamine remains an important but unanswered question.
Another putative neurochemical that may be of importance to STN DBS mechanisms is adenosine. Proposed as a chemical mediator of thalamic DBS for the treatment of ET,174 adenosine release can be measured in the striatum with chemical microsensors during electrical stimulation in the vicinity of the nigrostriatal dopaminergic tract.175,176 Importantly, increases in extracellular adenosine appear to match elevations in CBF resulting from increases in neural activity.177 Indeed, we have demonstrated that STN DBS elicits adenosine release in the striatum as measured by chemical microsensors.178 Adenosine is also known to play a role in astrocyte signaling and this fact may become even more important in view of growing interest in the local effects of DBS on glial cells.174,179,180
Glial cells far outnumber neurons in the brain and have, in recent years, been shown to play an active role in synaptic communication.181 This tripartite synapse hypothesis (involving pre- and post-synaptic neuronal elements and glia) has caused a paradigm shift in how we approach the study of neurotransmitter release and action on neural network function (see review by Perea and Araque182). There is now evidence to suggest that DBS activates glial cells directly to elicit release of gliotransmitters that, in turn, have widespread effects on the tripartite synapse and the neuronal network. As opposed to the previously presumed local inhibitory action of DBS at the site of stimulation, the prevailing effect appears to be excitation of both glial and neuronal elements and subsequent changes in neural network activity. Despite growing acceptance of this general scheme, the underlying questions of what elements are affected, how they are affected, and which neurotransmitters mediate these changes, remain largely unanswered.
It is well-documented that HFS modulates astrocyte activity by triggering the onset of a propagating Ca2+ wave.183-185 In fact, initial studies of astrocyte-neuronal interactions used electrical stimulation to evoke long-distance Ca2+ signaling186 and it is well known that electrical stimulation of brain tissue results in glial activation to increase intracellular cytosolic Ca2+ concentrations.187 Moreover, this local activation of glia can lead to a wave of Ca2+ influx that propagates through the glial cell syncytium in the brain for distances as great as several centimeters.187,188 Astrocytic Ca2+ increases, in turn, evoke the release of gliotransmitters, including ATP/adenosine, glutamate, D-serine, and PGE2.189-191 Release of these gliotransmitters can result in excitation or inhibition of neurons as well as the modulation of synaptic transmission and synaptic plasticity.181,186,192,193
Several studies have now established that astrocytes contain vesicular glutamate stores that can be triggered to undergo exocytosis by mechanical stimulation,194 in turn activating ionotropic195 or metabotropic glutamate receptors.196 In a similar manner, HFS of hippocampal slices or astrocyte cultures can elicit astrocytic Ca2+ waves.193,197 Astrocytes also release glutamate through volume-sensitive channels after ATP stimulation.198 This implies that specific stimuli may recruit different gliotransmitter release mechanisms to modify the spatio-temporal characteristics of subsequent neuronal responses. These and other studies have pointed to glutamate and adenosine as key mediators of astrocyte-to-neuron signaling.199 Astrocytes have also been implicated in mediating heterosynaptic depression, through the release of ATP and its subsequent catabolism to adenosine.174,200-202 Most notably, astrocytes respond to neuronal activity with waves of Ca2+ influx,203,204 which in turn elicit further glutamate release.205 Thus, DBS-induced Ca2+ signaling in astrocytes may affect neuronal network activity through gliotransmitter release, thereby playing an important role in the therapeutic mechanism of DBS.
DBS applied to the area containing tremor cells leads to immediate tremor arrest, an effect that is rapidly reversed when stimulation ceases.206 Similarly, we and others have demonstrated that DBS of the thalamus207,208 and STN209 results in neurotransmitter release, including glutamate. In our experimental condition, HFS applied to the thalamus led to immediate glutamate and adenosine release, which decreased to pre-stimulation levels when stimulation ceased.180 Thus, HFS-mediated glutamate and adenosine release may be important in the ability of DBS to abolish synchronized neural network oscillations such as those seen in tremor and seizures. Importantly, Bekar et al.174 have shown that thalamic DBS is associated with a marked increase in the local efflux of ATP and extracellular accumulation of its catabolic product, adenosine, which can act as a neuromodulator. Together, these findings suggest that DBS may activate multiple neurotransmitter systems, including glutamate and adenosine. Which of these neurotransmitter systems is primarily responsible for the effects of DBS in human patients and whether they are glial or neuronal in origin requires further investigation.
We and others contend that characterization of the hitherto understudied glial effects of DBS on neurotransmission will provide a deeper understanding of its corrective actions on dysfunctional brain processing, and consequently enhance our capacity to utilize its therapeutic effects in the patient.174,180 This basic knowledge will substantially enhance our potential to further develop DBS technology and surgical procedures to produce significant improvement in patient outcome. In particular, an integrative approach will be important in defining the causal relationships between DBS-mediated glial activation and neural network activity within the thalamus.
To address the scientific and clinical issues outlined so far, it is clear that more sophisticated brain imaging and real-time in vivo electrophysiological and neurochemical monitoring techniques will be essential to mechanistic studies of DBS action, optimizing electrode placement during surgery and in the future development of intelligent, closed-loop DBS systems. As such, we will now explore these areas in turn and outline their potential roles in the evolution of DBS therapy in the future.
Recent advances have dramatically increased the magnetic field strength for MRI, and prototype research systems have been fitted with 7.0 T magnets, which are five-times the strength of the typical 1.5 T machines currently used in most hospitals. High-contrast and high-resolution brain images can thus be obtained with an ultra high-field MRI system to provide details never before observed in the living human brain.210-212 Fig. 1 shows sagittal and coronal images collected by 7.0 T MRI. The detailed brain structures, in particular, the high contrast visualization of the STN, should improve the accuracy of determining stereotactic coordinates for positioning stimulation electrodes during DBS surgery.213
In addition to imaging, counter-localization of the target electrode site is currently performed using microelectrode electrophysiological recordings, but in the future this may be optimized by real-time neurochemical monitoring modalities (discussed below) together with analysis of local electrophysiological parameters. In terms of the latter, recently Zaidel et al.214 in a study of 128 PD patients have shown that optimal clinical outcome of bilateral STN DBS is correlated with placement of the electrode within a distinct dorsolateral oscillatory region of the STN (characterized by increased β-oscillatory activity on multi-unit recordings), rather than simply at its anatomical centre.
Importantly, another group have shown the benefits of subsequently tailoring stimulation parameters according to the final position of the DBS electrode. In the studies by Paek et al.215 and Lee et al.216 pre-operative 1.5 T MRI images were fused with either post-operative MRI (at 3 and 6 months) and post-operative CT (at 6 months) scans respectively and the three-dimensional anatomical location of electrode contacts used to re-program stimulation parameters. In both studies, there was a significant improvement in UPDRS-III motor subscale (in both on- and off-medication states) and reduction in medication requirements.
Finally, measuring the latency of saccadic eye movements in the conscious patient (saccadometry) may also facilitate optimal electrode targeting. Temel et al.217 have shown that saccadic latency is a sensitive and objective measure of the therapeutic effect of STN DBS and it correlates well with the UPDRS-III. In the future, this could form the basis of another quantitative mode of intra-operative guidance for electrode placement in the STN.
Microdialysis and voltammetry are the two most widely used techniques for neurochemical monitoring in vivo.218,219 However, recording from the basal ganglia complex during clinically-relevant DBS of the STN requires a degree of temporal and spatial sampling analysis that voltammetric techniques easily provide over conventional microdialysis procedures (i.e., subsecond time scales and µm-sized space domains220). For our own aims of examining the functional anatomical and neurochemical effects of STN DBS, we developed the Wireless Instantaneous Neurotransmitter Concentration System (WINCS)-a device designed specifically to monitor neurochemical release during experimental and clinical DBS surgery. As such, research subject safety, signal fidelity, and integration with existing DBS surgical procedures, and now MRI pre-, intra, and postoperative analysis, have been key priorities during the development of WINCS. Additionally, it is easily attached to the stereotactic frame and transmits neurochemical release data to a remotely located base station (within 10 m), thus facilitating recording parameter settings and data acquisition, while minimizing encumbrance of personnel and equipment at the operating/recording site.
The WINCS device, designed in compliance with FDA-recognized consensus standards for medical electrical device safety, consists of a relatively small, wireless, sterilizable battery-powered unit that can interface with carbon-fiber microelectrodes (CFM) or enzyme-based microsensors for real-time monitoring of neurotransmitter release in mammalian brain.221-223 Indeed, our own work in pigs has utilized fast scan cyclic voltammetry (FSCV) and CFMs to evaluate neurotransmission within the basal ganglia during STN DBS. Briefly, FSCV employs a linearly scanned potential (V) applied at 100 ms intervals to brain-implanted CFMs and compared to an Ag/AgCl reference electrode in contact with the cortical surface of the brain. This detection scheme generates a voltammogram (a plot of measured oxidation and reduction current versus the applied potential) that provides a signature to identify the recorded chemical (Fig. 2). Changes in the amplitude of the oxidation peak recorded by FSCV thus provide a quantitative concentration measurement of the temporal effects of electrical stimulation on neurochemical release. Neurotransmitters and neuromodulators amenable to detection by FSCV include dopamine, serotonin, norepinephrine, adenosine, and nitric oxide.157,178,220,224-229
For our DBS studies, the WINCS device has significant advantages over other commercially available wireless recording systems as it offers 1) an advanced microprocessor with superior analog to digital conversion, greater internal memory, and faster clock speed, 2) wirelessly programmable waveform parameters (scan bias, range and rate) using an advanced Bluetooth® module for wireless communication, 3) a higher precision voltage reference for the micro-processor, 4) a low-power mode to preserve battery life, voltage sensing, and low-power alert, and most importantly 5) proven compatibility and functionality in the bore of an MRI during image acquisition. The engineered compatibility of WINCS with MRI offers a unique opportunity to quantify regional variations in neurotransmission by FSCV during fMRI procedures opening up exciting and novel research directions that go well beyond investigations of central DBS mechanisms.
DBS in its current open-loop form is always on and not guided by any changes in underlying brain activity related to the disorder being treated. However, given that the brain communicates both electrically and chemically, it is likely that the most effective treatments for many neurological and psychiatric disorders will, in the future, involve returning both electrical firing patterns and neurotransmitter release levels back to normal.230 NeuroPace (Mountain View, CA, USA) have developed a closed-loop responsive neurostimulation (RNS®) device for the treatment of refractory partial epilepsy that uses cortical surface (subdural) or depth electrodes sited at the seizure focus to monitor for abnormal electrical activity in order to elicit a pre-programmed normalizing burst of stimulation to prevent seizure onset. Alternatively, oscillations in the electrical field immediately around (and recorded using) DBS electrodes which reflect synchronized activity of large populations of neurons, termed local field potentials, have also been proposed as feedback sources for online adjustment of DBS parameters.231,232
While the ability to undertake intra- and post-operative neurochemical monitoring will be crucial in the short-term for testing predictions of the neurotransmitter release (synaptic modulation) hypothesis of DBS, in the long-term it too may form a blueprint for a closed-loop DBS device supporting all-in-one neuromonitoring and neuromodulation. Conceivably, a neuroprosthesis supporting real-time, instantaneous neurochemical sensing and feedback-guided adjustment of stimulation to maintain therapeutic neurotransmitter levels would be superior to drug treatments for neuropsychiatric disorders that entail onset and offset effects. Indeed, we have initiated work towards the ultimate realization of a closed-loop smart DBS device utilizing this rationale. One critical component is an ultra-small, low-power integrated circuit supporting wireless neurochemical monitoring. By using very-large-scale-integration techniques in standard complementary-metal-oxide-semiconductor technology, we have been able to fabricate a wireless device, with dimensions of 2.2×2.2 mm, supporting single-channel FSCV. Indeed, this device compared favorably to a conventional hardwired system in calibration tests in vitro and for measuring electrical stimulation-evoked dopamine levels in the anesthetized rat.233
DBS is now a widely accepted and efficacious therapy for many conditions, but we still lack a definitive understanding of its mechanism of action. Indeed, while the nature of DBS devices make them amenable to blinded trials aimed at testing the efficacy of HFS in emerging clinical contexts, future paradigm shifts in DBS technology and the movement towards closed-loop devices undoubtedly rest on our ability to gain a clearer understanding of the mechanisms responsible for the clinical effect of DBS. Fortunately, it appears that the same technological advances demanded by basic science investigators may also turn out to be central to the design of future intelligent DBS systems used by clinicians.
Figures and Tables
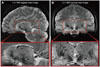 | Fig. 1Sagittal (A) and coronal (B) images obtained by 7.0 T MRI using a brain-optimized sensitivity encoding coil. Areas shown are the most complex areas in the brain with numerous nuclei readily visible, including subthalamic nucleus (STN), substantia nigra (SN), claustrum (Cl), putamen (Pu), globus pallidus externa and interna (GPe and GPi), posterior cerebral artery (PCA), third ventricle (3V), and hippocampus (HC) among others. |
 | Fig. 2Plots showing wireless detection of adenosine using WINCS at a CFM in vitro. A: Pseudocolor plot obtained during a 20 second flow cell injection of 5 µM adenosine, exhibiting 3D information. The x axis, y axis, and color gradient indicate time, voltage applied at the CFM, and current (I) detected from the CFM, respectively. The FSCV waveform was applied from -0.4 V to +1.5 V and back to -0.4 V at 400 V/second every 100 msec. A green oval surrounded by a purple ring first appears around +1.5 V after the adenosine injection, and this represents the first oxidative peak of adenosine. A second oxidative peak around +1.0 V occurs after the appearance of the first oxidative peak. B: Graph showing current versus time traces for the first and second peak oxidative currents (taken along horizontal black and red dotted lines respectively on 2A). C: A representative background-subtracted folded voltammogram of adenosine, showing 1st and 2nd oxidative peaks (taken along vertical solid black line in 2A). D: Picture of the WINCS device chipset relative to a United States quarter dollar coin. WINCS: Wireless Instantaneous Neurotransmitter Concentration System, CFM: carbon-fiber microelectrodes, FSCV: fast scan cyclic voltammetry. |
Acknowledgements
We wish to recognize and thank Kevin E. Bennet, April E. Horne, Christopher J. Kimble, David M. Johnson, Kenneth R. Kressin, Justin C. Robinson, Sidney V. Whitlock and Bruce A. Winter of the Division of Engineering at Mayo Clinic for their invaluable efforts in the realization of the WINCS device and software.
This work was supported by NIH (K08 NS 52232 award) and Mayo Foundation (2008-2010 Research Early Career Development Award for Clinician Scientists) to KHL.
References
1. Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003. 349:1925–1934.

2. Bereznai B, Steude U, Seelos K, Bötzel K. Chronic high-frequency globus pallidus internus stimulation in different types of dystonia: a clinical, video, and MRI report of six patients presenting with segmental, cervical, and generalized dystonia. Mov Disord. 2002. 17:138–144.

3. Koller WC, Pahwa PR, Lyons KE, Wilkinson SB. Deep brain stimulation of the Vim nucleus of the thalamus for the treatment of tremor. Neurology. 2000. 55:S29–S33.
6. Lipsman N, Neimat JS, Lozano AM. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: the search for a valid target. Neurosurgery. 2007. 61:1–11. discussion 11-13.
7. Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006. 31:2384–2393.

8. Hardesty DE, Sackeim HA. Deep brain stimulation in movement and psychiatric disorders. Biol Psychiatry. 2007. 61:831–835.

9. Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005. 45:651–660.

10. Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008. 33:368–377.

11. Vonck K, Boon P, Goossens L, Dedeurwaerdere S, Claeys P, Gossiaux F, et al. Neurostimulation for refractory epilepsy. Acta Neurol Belg. 2003. 103:213–217.
12. Boon P, Vonck K, De Herdt V, Van Dycke A, Goethals M, Goossens L, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007. 48:1551–1560.

13. Hodaie M, Wennberg RA, Dostrovsky JO, Lozano AM. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002. 43:603–608.

14. Maciunas RJ, Maddux BN, Riley DE, Whitney CM, Schoenberg MR, Ogrocki PJ, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007. 107:1004–1014.

15. Rasche D, Rinaldi PC, Young RF, Tronnier VM. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Focus. 2006. 21:E8.

16. Bittar RG, Kar-Purkayastha I, Owen SL, Bear RE, Green A, Wang S, et al. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005. 12:515–519.

17. Olanow CW. Levodopa/dopamine replacement strategies in Parkinson's disease--future directions. Mov Disord. 2008. 23:Suppl 3. S613–S622.

19. Nagatsua T, Sawadab M. L-dopa therapy for Parkinson's disease: past, present, and future. Parkinsonism Relat Disord. 2009. 15:Suppl 1. S3–S8.
20. Remple MS, Sarpong Y, Neimat JS. Frontiers in the surgical treatment of Parkinson's disease. Expert Rev Neurother. 2008. 8:897–906.

21. Nandhagopal R, McKeown MJ, Stoessl AJ. Functional imaging in Parkinson disease. Neurology. 2008. 70:1478–1488.
22. Poewe W. Treatments for Parkinson disease--past achievements and current clinical needs. Neurology. 2009. 72:S65–S73.

23. Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009. 301:63–73.

24. Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006. 355:896–908.

25. Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 2010. 9:581–591.

26. Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 1998. 339:1105–1111.

27. Volkmann J, Allert N, Voges J, Weiss PH, Freund HJ, Sturm V. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology. 2001. 56:548–551.

28. Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991. 337:403–406.

29. Koller W, Pahwa R, Busenbark K, Hubble J, Wilkinson S, Lang A, et al. High-frequency unilateral thalamic stimulation in the treatment of essential and parkinsonian tremor. Ann Neurol. 1997. 42:292–299.

30. Tasker RR. Deep brain stimulation is preferable to thalamotomy for tremor suppression. Surg Neurol. 1998. 49:145–153. discussion 153-154.

31. Limousin P, Speelman JD, Gielen F, Janssens M. Multicentre European study of thalamic stimulation in parkinsonian and essential tremor. J Neurol Neurosurg Psychiatry. 1999. 66:289–296.

32. Rehncrona S, Johnels B, Widner H, Törnqvist AL, Hariz M, Sydow O. Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov Disord. 2003. 18:163–170.

33. Burchiel KJ, Anderson VC, Favre J, Hammerstad JP. Comparison of pallidal and subthalamic nucleus deep brain stimulation for advanced Parkinson's disease: results of a randomized, blinded pilot study. Neurosurgery. 1999. 45:1375–1382. discussion 1382-1384.

34. Obeso JA, Marin C, Rodriguez-Oroz C, Blesa J, Benitez-Temiño B, Mena-Segovia J, et al. The basal ganglia in Parkinson's disease: current concepts and unexplained observations. Ann Neurol. 2008. 64:Suppl 2. S30–S46.

35. Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain. 2005. 128:2240–2249.

36. Ghika J, Villemure JG, Fankhauser H, Favre J, Assal G, Ghika-Schmid F. Efficiency and safety of bilateral contemporaneous pallidal stimulation (deep brain stimulation) in levodopa-responsive patients with Parkinson's disease with severe motor fluctuations: a 2-year follow-up review. J Neurosurg. 1998. 89:713–718.

37. Volkmann J, Sturm V, Weiss P, Kappler J, Voges J, Koulousakis A, et al. Bilateral high-frequency stimulation of the internal globus pallidus in advanced Parkinson's disease. Ann Neurol. 1998. 44:953–961.

38. Kumar R, Lang AE, Rodriguez-Oroz MC, Lozano AM, Limousin P, Pollak P, et al. Deep brain stimulation of the globus pallidus pars interna in advanced Parkinson's disease. Neurology. 2000. 55:S34–S39.
39. Lyons KE, Wilkinson SB, Tröster AI, Pahwa R. Long-term efficacy of globus pallidus stimulation for the treatment of Parkinson's disease. Stereotact Funct Neurosurg. 2002. 79:214–220.

40. Loher TJ, Burgunder JM, Weber S, Sommerhalder R, Krauss JK. Effect of chronic pallidal deep brain stimulation on off period dystonia and sensory symptoms in advanced Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002. 73:395–399.

41. Rodrigues JP, Walters SE, Watson P, Stell R, Mastaglia FL. Globus pallidus stimulation in advanced Parkinson's disease. J Clin Neurosci. 2007. 14:208–215.

42. Durif F, Lemaire JJ, Debilly B, Dordain G. Long-term follow-up of globus pallidus chronic stimulation in advanced Parkinson's disease. Mov Disord. 2002. 17:803–807.

43. Grasso R, Peppe A, Stratta F, Angelini D, Zago M, Stanzione P, et al. Basal ganglia and gait control: apomorphine administration and internal pallidum stimulation in Parkinson's disease. Exp Brain Res. 1999. 126:139–148.

44. Volkmann J, Allert N, Voges J, Sturm V, Schnitzler A, Freund HJ. Long-term results of bilateral pallidal stimulation in Parkinson's disease. Ann Neurol. 2004. 55:871–875.

45. Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med. 2010. 362:2077–2091.

46. Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain. 2010. 133:215–224.

47. Ferraye MU, Debû B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson's disease. Brain. 2010. 133:205–214.

48. Bain PG, Findley LJ, Thompson PD, Gresty MA, Rothwell JC, Harding AE, et al. A study of hereditary essential tremor. Brain. 1994. 117:805–824.

49. Louis ED, Thawani SP, Andrews HF. Prevalence of essential tremor in a multiethnic, community-based study in northern Manhattan, New York, N.Y. Neuroepidemiology. 2009. 32:208–214.

50. Benabid AL, Pollak P, Seigneuret E, Hoffmann D, Gay E, Perret J. Chronic VIM thalamic stimulation in Parkinson's disease, essential tremor and extra-pyramidal dyskinesias. Acta Neurochir Suppl (Wien). 1993. 58:39–44.

51. Schuurman PR, Bosch DA, Bossuyt PM, Bonsel GJ, van Someren EJ, de Bie RM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000. 342:461–468.

52. Sydow O, Thobois S, Alesch F, Speelman JD. Multicentre European study of thalamic stimulation in essential tremor: a six year follow up. J Neurol Neurosurg Psychiatry. 2003. 74:1387–1391.

53. Putzke JD, Uitti RJ, Obwegeser AA, Wszolek ZK, Wharen RE. Bilateral thalamic deep brain stimulation: midline tremor control. J Neurol Neurosurg Psychiatry. 2005. 76:684–690.

54. Taha JM, Janszen MA, Favre J. Thalamic deep brain stimulation for the treatment of head, voice, and bilateral limb tremor. J Neurosurg. 1999. 91:68–72.

55. Aziz TZ, Green AL. Dystonia: a surgeon's perspective. Parkinsonism Relat Disord. 2009. 15:Suppl 3. S75–S80.

57. Isaias IU, Alterman RL, Tagliati M. Deep brain stimulation for primary generalized dystonia: long-term outcomes. Arch Neurol. 2009. 66:465–470.
58. Sako W, Goto S, Shimazu H, Murase N, Matsuzaki K, Tamura T, et al. Bilateral deep brain stimulation of the globus pallidus internus in tardive dystonia. Mov Disord. 2008. 23:1929–1931.

59. Kefalopoulou Z, Paschali A, Markaki E, Vassilakos P, Ellul J, Constantoyannis C. A double-blind study on a patient with tardive dyskinesia treated with pallidal deep brain stimulation. Acta Neurol Scand. 2009. 119:269–273.

60. Damier P, Thobois S, Witjas T, Cuny E, Derost P, Raoul S, et al. Bilateral deep brain stimulation of the globus pallidus to treat tardive dyskinesia. Arch Gen Psychiatry. 2007. 64:170–176.

61. Kuhn J, Gründler TO, Lenartz D, Sturm V, Klosterkötter J, Huff W. Deep brain stimulation for psychiatric disorders. Dtsch Arztebl Int. 2010. 107:105–113.

62. Tye SJ, Frye MA, Lee KH. Disrupting disordered neurocircuitry: treating refractory psychiatric illness with neuromodulation. Mayo Clin Proc. 2009. 84:522–532.

63. Ward HE, Hwynn N, Okun MS. Update on deep brain stimulation for neuropsychiatric disorders. Neurobiol Dis. 2010. 38:346–353.

64. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005. 62:617–627.

65. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000. 157:1–45.
66. Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999. 156:675–682.
67. Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004. 22:409–418.

68. Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000. 48:830–843.

69. Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell CC, Sackeim HA, et al. Decreased regional brain metabolism after ect. Am J Psychiatry. 2001. 158:305–308.

70. Mottaghy FM, Keller CE, Gangitano M, Ly J, Thall M, Parker JA, et al. Correlation of cerebral blood flow and treatment effects of repetitive transcranial magnetic stimulation in depressed patients. Psychiatry Res. 2002. 115:1–14.

71. Dougherty DD, Weiss AP, Cosgrove GR, Alpert NM, Cassem EH, Nierenberg AA, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg. 2003. 99:1010–1017.

72. Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008. 64:461–467.

73. Hauptman JS, DeSalles AA, Espinoza R, Sedrak M, Ishida W. Potential surgical targets for deep brain stimulation in treatment-resistant depression. Neurosurg Focus. 2008. 25:E3.

74. Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010. 67:110–116.

75. Van Laere K, Nuttin B, Gabriels L, Dupont P, Rasmussen S, Greenberg BD, et al. Metabolic imaging of anterior capsular stimulation in refractory obsessive-compulsive disorder: a key role for the subgenual anterior cingulate and ventral striatum. J Nucl Med. 2006. 47:740–747.
76. Cosyns P, Gabriels L, Nuttin B. Deep brain stimulation in treatment refractory obsessive compulsive disorder. Verh K Acad Geneeskd Belg. 2003. 65:385–399. discussion 399-400.
77. Nuttin BJ, Gabriels L, van Kuyck K, Cosyns P. Electrical stimulation of the anterior limbs of the internal capsules in patients with severe obsessive-compulsive disorder: anecdotal reports. Neurosurg Clin N Am. 2003. 14:267–274.

78. Nuttin BJ, Gabriëls LA, Cosyns PR, Meyerson BA, Andréewitch S, Sunaert SG, et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003. 52:1263–1272. discussion 1272-1274.

79. Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999. 354:1526.

80. Gabriëls L, Cosyns P, Nuttin B, Demeulemeester H, Gybels J. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: psychopathological and neuropsychological outcome in three cases. Acta Psychiatr Scand. 2003. 107:275–282.

81. Malone DA Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009. 65:267–275.

83. Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, et al. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive-and anxiety-disorders. J Chem Neuroanat. 2003. 26:293–299.

84. Huff W, Lenartz D, Schormann M, Lee SH, Kuhn J, Koulousakis A, et al. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment-resistant obsessive-compulsive disorder: Outcomes after one year. Clin Neurol Neurosurg. 2010. 112:137–143.

85. Jung HH, Kim CH, Chang JH, Park YG, Chung SS, Chang JW. Bilateral anterior cingulotomy for refractory obsessive-compulsive disorder: Long-term follow-up results. Stereotact Funct Neurosurg. 2006. 84:184–189.

86. Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005. 57:510–516.

87. Mallet L, Mesnage V, Houeto JL, Pelissolo A, Yelnik J, Behar C, et al. Compulsions, Parkinson's disease, and stimulation. Lancet. 2002. 360:1302–1304.

88. Fontaine D, Mattei V, Borg M, von Langsdorff D, Magnie MN, Chanalet S, et al. Effect of subthalamic nucleus stimulation on obsessive-compulsive disorder in a patient with Parkinson disease. Case report. J Neurosurg. 2004. 100:1084–1086.

89. Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008. 359:2121–2134.

90. Ackermans L, Temel Y, Visser-Vandewalle V. Deep brain stimulation in Tourettes Syndrome. Neurotherapeutics. 2008. 5:339–344.

92. Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999. 353:724.

93. Welter ML, Mallet L, Houeto JL, Karachi C, Czernecki V, Cornu P, et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol. 2008. 65:952–957.

94. Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry. 2008. 79:136–142.

95. Porta M, Brambilla A, Cavanna AE, Servello D, Sassi M, Rickards H, et al. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009. 73:1375–1380.

96. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000. 342:314–319.

97. Kahane P, Depaulis A. Deep brain stimulation in epilepsy: what is next? Curr Opin Neurol. 2010. 23:177–182.

98. Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010. 51:899–908.

99. Coffey RJ. Deep brain stimulation for chronic pain: results of two multicenter trials and a structured review. Pain Med. 2001. 2:183–192.

100. Hamani C, Schwalb JM, Rezai AR, Dostrovsky JO, Davis KD, Lozano AM. Deep brain stimulation for chronic neuropathic pain: long-term outcome and the incidence of insertional effect. Pain. 2006. 125:188–196.

101. Bittar RG, Otero S, Carter H, Aziz TZ. Deep brain stimulation for ph-antom limb pain. J Clin Neurosci. 2005. 12:399–404.

102. Pereira EA, Green AL, Bradley KM, Soper N, Moir L, Stein JF, et al. Regional cerebral perfusion differences between periventricular grey, thalamic and dual target deep brain stimulation for chronic neuropathic pain. Stereotact Funct Neurosurg. 2007. 85:175–183.

103. Owen SL, Green AL, Stein JF, Aziz TZ. Deep brain stimulation for the alleviation of post-stroke neuropathic pain. Pain. 2006. 120:202–206.

104. Franzini A, Ferroli P, Leone M, Broggi G. Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported series. Neurosurgery. 2003. 52:1095–1099. discussion 1099-1101.

105. Schoenen J, Di Clemente L, Vandenheede M, Fumal A, De Pasqua V, Mouchamps M, et al. Hypothalamic stimulation in chronic cluster he-adache: a pilot study of efficacy and mode of action. Brain. 2005. 128:940–947.

106. Leone M, Franzini A, Broggi G, Bussone G. Hypothalamic stimulation for intractable cluster headache: long-term experience. Neurology. 2006. 67:150–152.

107. Green AL, Owen SL, Davies P, Moir L, Aziz TZ. Deep brain stimulation for neuropathic cephalalgia. Cephalalgia. 2006. 26:561–567.

108. Koike Y, Shima F, Nakamizo A, Miyagi Y. Direct localization of subthalamic nucleus supplemented by single-track electrophysiological guidance in deep brain stimulation lead implantation: techniques and clinical results. Stereotact Funct Neurosurg. 2008. 86:173–178.

109. Shin M, Lefaucheur JP, Penholate MF, Brugières P, Gurruchaga JM, Nguyen JP. Subthalamic nucleus stimulation in Parkinson's disease: postoperative CT-MRI fusion images confirm accuracy of electrode placement using intraoperative multi-unit recording. Neurophysiol Clin. 2007. 37:457–466.

110. Binder DK, Rau G, Starr PA. Hemorrhagic complications of microelectrode-guided deep brain stimulation. Stereotact Funct Neurosurg. 2003. 80:28–31.

111. Bezerra ML, Martínez JV, Nasser JA. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med. 1999. 341:1003. author reply 1004.

112. Berney A, Vingerhoets F, Perrin A, Guex P, Villemure JG, Burkhard PR, et al. Effect on mood of subthalamic DBS for Parkinson's disease: a consecutive series of 24 patients. Neurology. 2002. 59:1427–1429.

113. Patel NK, Heywood P, O'Sullivan K, McCarter R, Love S, Gill SS. Unilateral subthalamotomy in the treatment of Parkinson's disease. Brain. 2003. 126:1136–1145.

114. Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990. 249:1436–1438.

115. Benabid AL, Koudsié A, Benazzouz A, Fraix V, Ashraf A, Le Bas JF, et al. Subthalamic stimulation for Parkinson's disease. Arch Med Res. 2000. 31:282–289.

116. Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987. 50:344–346.

117. Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol. 2001. 85:1351–1356.

118. Magariños-Ascone C, Pazo JH, Macadar O, Buño W. High-frequency stimulation of the subthalamic nucleus silences subthalamic neurons: a possible cellular mechanism in Parkinson's disease. Neuroscience. 2002. 115:1109–1117.

119. Garcia L, D'Alessandro G, Bioulac B, Hammond C. High-frequency stimulation in Parkinson's disease: more or less? Trends Neurosci. 2005. 28:209–216.

120. McIntyre CC, Grill WM. Sensitivity analysis of a model of mammalian neural membrane. Biol Cybern. 1998. 79:29–37.

121. McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 2004. 91:1457–1469.

122. McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004. 115:1239–1248.

123. McIntyre CC, Savasta M, Walter BL, Vitek JL. How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol. 2004. 21:40–50.

124. Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004. 15:1137–1140.

125. Johnson MD, Miocinovic S, McIntyre CC, Vitek JL. Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics. 2008. 5:294–308.

126. Miocinovic S, Parent M, Butson CR, Hahn PJ, Russo GS, Vitek JL, et al. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. J Neurophysiol. 2006. 96:1569–1580.

127. Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003. 23:1916–1923.

128. Kita H, Tachibana Y, Nambu A, Chiken S. Balance of monosynaptic excitatory and disynaptic inhibitory responses of the globus pallidus induced after stimulation of the subthalamic nucleus in the monkey. J Neurosci. 2005. 25:8611–8619.

129. Smith ID, Grace AA. Role of the subthalamic nucleus in the regulation of nigral dopamine neuron activity. Synapse. 1992. 12:287–303.

130. Benazzouz A, Gao D, Ni Z, Benabid AL. High frequency stimulation of the STN influences the activity of dopamine neurons in the rat. Neuroreport. 2000. 11:1593–1596.

131. Maurice N, Thierry AM, Glowinski J, Deniau JM. Spontaneous and evoked activity of substantia nigra pars reticulata neurons during high-frequency stimulation of the subthalamic nucleus. J Neurosci. 2003. 23:9929–9936.

132. Ceballos-Baumann AO. Functional imaging in Parkinson's disease: activation studies with PET, fMRI and SPECT. J Neurol. 2003. 250:Suppl 1. I15–I23.

133. Hershey T, Revilla FJ, Wernle AR, McGee-Minnich L, Antenor JV, Videen TO, et al. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003. 61:816–821.

134. Sestini S, Ramat S, Formiconi AR, Ammannati F, Sorbi S, Pupi A. Brain networks underlying the clinical effects of long-term subthalamic stimulation for Parkinson's disease: a 4-year follow-up study with rCBF SPECT. J Nucl Med. 2005. 46:1444–1454.
135. Eidelberg D, Edwards C. Functional brain imaging of movement disorders. Neurol Res. 2000. 22:305–312.

136. Grafton ST, DeLong M. Tracing the brains circuitry with functional imaging. Nat Med. 1997. 3:602–603.

137. Karimi M, Golchin N, Tabbal SD, Hershey T, Videen TO, Wu J, et al. Subthalamic nucleus stimulation-induced regional blood flow responses correlate with improvement of motor signs in Parkinson disease. Brain. 2008. 131:2710–2719.

138. Zhao YB, Sun BM, Li DY, Wang QS. Effects of bilateral subthalamic nucleus stimulation on resting-state cerebral glucose metabolism in advanced Parkinson's disease. Chin Med J (Engl). 2004. 117:1304–1308.
139. Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990. 87:9868–9872.

140. van Eijsden P, Hyder F, Rothman DL, Shulman RG. Neurophysiology of functional imaging. Neuroimage. 2009. 45:1047–1054.

141. Babiloni C, Pizzella V, Gratta CD, Ferretti A, Romani GL. Fundamentals of electroencefalography, magnetoencefalography, and functional magnetic resonance imaging. Int Rev Neurobiol. 2009. 86:67–80.
142. Jech R, Urgosík D, Tintera J, Nebuzelský A, Krásenský J, Liscák R, et al. Functional magnetic resonance imaging during deep brain stimulation: a pilot study in four patients with Parkinson's disease. Mov Disord. 2001. 16:1126–1132.

143. Phillips MD, Baker KB, Lowe MJ, Tkach JA, Cooper SE, Kopell BH, et al. Parkinson disease: pattern of functional MR imaging activation during deep brain stimulation of subthalamic nucleus--initial experience. Radiology. 2006. 239:209–216.

144. Stefurak T, Mikulis D, Mayberg H, Lang AE, Hevenor S, Pahapill P, et al. Deep brain stimulation for Parkinson's disease dissociates mood and motor circuits: a functional MRI case study. Mov Disord. 2003. 18:1508–1516.

146. Lang AE, Lozano AM. Parkinson's disease. Second of two parts. N Engl J Med. 1998. 339:1130–1143.
147. Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998. 339:1044–1053.
148. Gerlach M, van den Buuse M, Blaha C, Bremen D, Riederer P. Entacapone increases and prolongs the central effects of l-DOPA in the 6-hydroxydopamine-lesioned rat. Naunyn Schmiedebergs Arch Pharmacol. 2004. 370:388–394.

149. Moro E, Scerrati M, Romito LM, Roselli R, Tonali P, Albanese A. Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson's disease. Neurology. 1999. 53:85–90.

150. Molinuevo JL, Valldeoriola F, Tolosa E, Rumia J, Valls-Sole J, Roldan H, et al. Levodopa withdrawal after bilateral subthalamic nucleus stimulation in advanced Parkinson disease. Arch Neurol. 2000. 57:983–988.

151. Paul G, Reum T, Meissner W, Marburger A, Sohr R, Morgenstern R, et al. High frequency stimulation of the subthalamic nucleus influences striatal dopaminergic metabolism in the naive rat. Neuroreport. 2000. 11:441–444.

152. Meissner W, Reum T, Paul G, Harnack D, Sohr R, Morgenstern R, et al. Striatal dopaminergic metabolism is increased by deep brain stimulation of the subthalamic nucleus in 6-hydroxydopamine lesioned rats. Neurosci Lett. 2001. 303:165–168.

153. Meissner W, Harnack D, Paul G, Reum T, Sohr R, Morgenstern R, et al. Deep brain stimulation of subthalamic neurons increases striatal dopamine metabolism and induces contralateral circling in freely moving 6-hydroxydopamine-lesioned rats. Neurosci Lett. 2002. 328:105–108.

154. Windels F, Bruet N, Poupard A, Urbain N, Chouvet G, Feuerstein C, et al. Effects of high frequency stimulation of subthalamic nucleus on extracellular glutamate and GABA in substantia nigra and globus pallidus in the normal rat. Eur J Neurosci. 2000. 12:4141–4146.

155. Windels F, Bruet N, Poupard A, Feuerstein C, Bertrand A, Savasta M. Influence of the frequency parameter on extracellular glutamate and gamma-aminobutyric acid in substantia nigra and globus pallidus during electrical stimulation of subthalamic nucleus in rats. J Neurosci Res. 2003. 72:259–267.

156. Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. J Neurosci Methods. 1999. 90:129–142.

157. Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003. 49:1763–1773.

158. Borland LM, Shi G, Yang H, Michael AC. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J Neurosci Methods. 2005. 146:149–158.

159. Lee KH, Blaha CD, Harris BT, Cooper S, Hitti FL, Leiter JC, et al. Dopamine efflux in the rat striatum evoked by electrical stimulation of the subthalamic nucleus: potential mechanism of action in Parkinson's disease. Eur J Neurosci. 2006. 23:1005–1014.

160. Blaha CD, Lester DB, Ramsson ES, Lee KH, Garris PA. Striatal dopamine release evoked by subthalamic stimulation in intact and 6-OHDA-lesioned rats: relevance to deep brain stimulation in Parkinson's Disease. Monitoring Molecules in Neuroscience. 2008. 395–397.
161. Covey DP, Ramsson ES, Heidenreich BA, Blaha CD, Lee KH, Garris PA. Monitoring subthalamic nucleus-evoked dopamine release in the striatum using fast-scan cyclic voltammetry in vivo. Monitoring Molecules in Neuroscience. 2008.
162. Shon YM, Lee KH, Goerss SJ, Kim IY, Kimble C, Van Gompel JJ, et al. High frequency stimulation of the subthalamic nucleus evokes striatal dopamine release in a large animal model of human DBS neurosurgery. Neurosci Lett. 2010. 475:136–140.

163. Meissner W, Harnack D, Reese R, Paul G, Reum T, Ansorge M, et al. High-frequency stimulation of the subthalamic nucleus enhances striatal dopamine release and metabolism in rats. J Neurochem. 2003. 85:601–609.

164. Bruet N, Windels F, Bertrand A, Feuerstein C, Poupard A, Savasta M. High frequency stimulation of the subthalamic nucleus increases the extracellular contents of striatal dopamine in normal and partially dopaminergic denervated rats. J Neuropathol Exp Neurol. 2001. 60:15–24.

165. Abosch A, Kapur S, Lang AE, Hussey D, Sime E, Miyasaki J, et al. Stimulation of the subthalamic nucleus in Parkinson's disease does not produce striatal dopamine release. Neurosurgery. 2003. 53:1095–1102. discussion 1102-1105.

166. Hilker R, Voges J, Ghaemi M, Lehrke R, Rudolf J, Koulousakis A, et al. Deep brain stimulation of the subthalamic nucleus does not increase the striatal dopamine concentration in parkinsonian humans. Mov Disord. 2003. 18:41–48.

167. Thobois S, Fraix V, Savasta M, Costes N, Pollak P, Mertens P, et al. Chronic subthalamic nucleus stimulation and striatal D2 dopamine receptors in Parkinson's disease--A [(11)C]-raclopride PET study. J Neurol. 2003. 250:1219–1223.

168. Strafella AP, Sadikot AF, Dagher A. Subthalamic deep brain stimulation does not induce striatal dopamine release in Parkinson's disease. Neuroreport. 2003. 14:1287–1289.

169. Volkow ND, Fowler JS, Wang GJ, Dewey SL, Schlyer D, MacGregor R, et al. Reproducibility of repeated measures of carbon-11-raclopride binding in the human brain. J Nucl Med. 1993. 34:609–613.
170. Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000. 20:423–451.

173. Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007. 318:1309–1312.

174. Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, et al. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med. 2008. 14:75–80.

175. Cechova S, Venton BJ. Transient adenosine efflux in the rat caudate-putamen. J Neurochem. 2008. 105:1253–1263.

176. Phillis JW. Adenosine and adenine nucleotides as regulators of cerebral blood flow: roles of acidosis, cell swelling, and KATP channels. Crit Rev Neurobiol. 2004. 16:237–270.

177. Brundege JM, Dunwiddie TV. Role of adenosine as a modulator of synaptic activity in the central nervous system. Adv Pharmacol. 1997. 39:353–391.

178. Shon YM, Chang SY, Tye SJ, Kimble CJ, Bennet KE, Blaha CD, et al. Comonitoring of adenosine and dopamine using the Wireless Instantaneous Neurotransmitter Concentration System: proof of principle. J Neurosurg. 2010. 112:539–548.

180. Tawfik VL, Chang SY, Hitti FL, Roberts DW, Leiter JC, Jovanovic S, et al. Deep brain stimulation results in local glutamate and adenosine release: investigation into the role of astrocytes. Neurosurgery. 2010. 67:367–375.

181. Bezzi P, Domercq M, Vesce S, Volterra A. Neuron-astrocyte cross-talk during synaptic transmission: physiological and neuropathological implications. Prog Brain Res. 2001. 132:255–265.

183. Bowser DN, Khakh BS. ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J Neurosci. 2004. 24:8606–8620.

184. Rossi D, Brambilla L, Valori CF, Crugnola A, Giaccone G, Capobianco R, et al. Defective tumor necrosis factor-alpha-dependent control of astrocyte glutamate release in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2005. 280:42088–42096.

185. Charles A. Reaching out beyond the synapse: glial intercellular waves coordinate metabolism. Sci STKE. 2005. 2005:pe6.

186. Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994. 263:1768–1771.

188. Zahs KR, Newman EA. Asymmetric gap junctional coupling between glial cells in the rat retina. Glia. 1997. 20:10–22.

189. Wang Z, Haydon PG, Yeung ES. Direct observation of calcium-independent intercellular ATP signaling in astrocytes. Anal Chem. 2000. 72:2001–2007.

190. Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001. 63:795–813.

192. Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003. 26:536–542.

193. Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992. 8:429–440.

194. Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000. 20:666–673.

195. Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J Neurosci. 2001. 21:477–484.

196. Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhäuser C, Pilati E, et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004. 7:613–620.

197. Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996. 16:5073–5081.

198. Takano T, Kang J, Jaiswal JK, Simon SM, Lin JH, Yu Y, et al. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc Natl Acad Sci U S A. 2005. 102:16466–16471.

199. Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci. 2002. 3:748–755.

200. Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, et al. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003. 40:971–982.

201. Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005. 310:113–116.

202. Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci. 2006. 26:5370–5382.

203. Hassinger TD, Atkinson PB, Strecker GJ, Whalen LR, Dudek FE, Kossel AH, et al. Evidence for glutamate-mediated activation of hippocampal neurons by glial calcium waves. J Neurobiol. 1995. 28:159–170.

204. Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994. 369:744–747.

205. Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, et al. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998. 391:281–285.

206. Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996. 84:203–214.

207. Lee KH, Hitti FL, Shalinsky MH, Kim U, Leiter JC, Roberts DW. Abolition of spindle oscillations and 3-Hz absence seizurelike activity in the thalamus by using high-frequency stimulation: potential mechanism of action. J Neurosurg. 2005. 103:538–545.

208. Anderson T, Hu B, Pittman Q, Kiss ZH. Mechanisms of deep brain stimulation: an intracellular study in rat thalamus. J Physiol. 2004. 559:301–313.

209. Lee KH, Kristic K, van Hoff R, Hitti FL, Blaha C, Harris B, et al. High-frequency stimulation of the subthalamic nucleus increases glutamate in the subthalamic nucleus of rats as demonstrated by in vivo enzyme-linked glutamate sensor. Brain Res. 2007. 1162:121–129.

210. Fischl B, Wald LL. Phase maps reveal cortical architecture. Proc Natl Acad Sci U S A. 2007. 104:11513–11514.

211. Duyn JH, van Gelderen P, Li TQ, de Zwart JA, Koretsky AP, Fukunaga M. High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci U S A. 2007. 104:11796–11801.

212. Cho ZH, Han JY, Hwang SI, Kim DS, Kim KN, Kim NB, et al. Quantitative analysis of the hippocampus using images obtained from 7.0 T MRI. Neuroimage. 2010. 49:2134–2140.

213. Cho ZH, Min HK, Oh SH, Han JY, Park CW, Chi JG, et al. Direct visualization of deep brain stimulation targets in Parkinson disease with the use of 7-tesla magnetic resonance imaging. J Neurosurg. 2010. 113:639–647.

214. Zaidel A, Spivak A, Grieb B, Bergman H, Israel Z. Subthalamic span of beta oscillations predicts deep brain stimulation efficacy for patients with Parkinson's disease. Brain. 2010. 133:2007–2021.

215. Paek SH, Han JH, Lee JY, Kim C, Jeon BS, Kim DG. Electrode position determined by fused images of preoperative and postoperative magnetic resonance imaging and surgical outcome after subthalamic nucleus deep brain stimulation. Neurosurgery. 2008. 63:925–936. discussion 936-927.

216. Lee JY, Jeon BS, Paek SH, Lim YH, Kim MR, Kim C. Reprogramming guided by the fused images of MRI and CT in subthalamic nucleus stimulation in Parkinson disease. Clin Neurol Neurosurg. 2010. 112:47–53.

217. Temel Y, Visser-Vandewalle V, Carpenter RH. Saccadometry: a novel clinical tool for quantification of the motor effects of subthalamic nucleus stimulation in Parkinson's disease. Exp Neurol. 2009. 216:481–489.

218. Blaha CD, Phillips AG. A critical assessment of electrochemical procedures applied to the measurement of dopamine and its metabolites during drug-induced and species-typical behaviours. Behav Pharmacol. 1996. 7:675–708.

219. Watson CJ, Venton BJ, Kennedy RT. In vivo measurements of neurotransmitters by microdialysis sampling. Anal Chem. 2006. 78:1391–1399.

220. Borland LM, Michael AC. Voltammetric study of the control of striatal dopamine release by glutamate. J Neurochem. 2004. 91:220–229.

221. Bledsoe JM, Kimble CJ, Covey DP, Blaha CD, Agnesi F, Mohseni P, et al. Development of the Wireless Instantaneous Neurotransmitter Concentration System for intraoperative neurochemical monitoring using fast-scan cyclic voltammetry. J Neurosurg. 2009. 111:712–723.

222. Agnesi F, Tye SJ, Bledsoe JM, Griessenauer CJ, Kimble CJ, Sieck GC, et al. Wireless Instantaneous Neurotransmitter Concentration System-based amperometric detection of dopamine, adenosine, and glutamate for intraoperative neurochemical monitoring. J Neurosurg. 2009. 111:701–711.

223. Shon YM, Chang SY, Tye SJ, Kimble CJ, Bennet KE, Blaha CD, et al. Comonitoring of adenosine and dopamine using the Wireless Instantaneous Neurotransmitter Concentration System: proof of principle. J Neurosurg. 2010. 112:539–548.

224. Agnesi F, Tye SJ, Bledsoe JM, Griessenauer CJ, Kimble CJ, Sieck GC, et al. Wireless Instantaneous Neurotransmitter Concentration System-based amperometric detection of dopamine, adenosine, and glutamate for intraoperative neurochemical monitoring. J Neurosurg. 2009. 111:701–711.

225. Griessenauer CJ, Chang SY, Tye SJ, Kimble CJ, Bennet KE, Garris PA, et al. Wireless Instantaneous Neurotransmitter Concentration System: electrochemical monitoring of serotonin using fast-scan cyclic voltammetry--a proof-of-principle study. J Neurosurg. 2010. 113:656–665.

226. Bledsoe JM, Kimble CJ, Covey DP, Blaha CD, Agnesi F, Mohseni P, et al. Development of the Wireless Instantaneous Neurotransmitter Concentration System for intraoperative neurochemical monitoring using fast-scan cyclic voltammetry. J Neurosurg. 2009. 111:712–723.

227. Mitchell KM. Acetylcholine and choline amperometric enzyme sensors characterized in vitro and in vivo. Anal Chem. 2004. 76:1098–1106.

228. Wilson GS, Gifford R. Biosensors for real-time in vivo measurements. Biosens Bioelectron. 2005. 20:2388–2403.

229. Sammut S, Park DJ, West AR. Frontal cortical afferents facilitate striatal nitric oxide transmission in vivo via a NMDA receptor and neuronal NOS-dependent mechanism. J Neurochem. 2007. 103:1145–1156.

230. Andrews RJ. Neuromodulation: advances in the next five years. Ann N Y Acad Sci. 2010. 1199:204–211.
231. Rossi L, Foffani G, Marceglia S, Bracchi F, Barbieri S, Priori A. An electronic device for artefact suppression in human local field potential recordings during deep brain stimulation. J Neural Eng. 2007. 4:96–106.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download