Abstract
Molecular imaging is a novel technology to visualize biological processes at the cellular and molecular levels, which is reshaping both biomedical research and clinical practice. By providing molecular information to supplement and augment conventional anatomy-based imaging, molecular imaging is expected to allow 1) the earlier detection of diseases, 2) precise evaluation of disease stages, and 3) both diagnostic and therapeutic monitoring of disease progression in a quantitative manner. In this brief review, we present our view on the prospects of molecular optical imaging in the field of stroke practice, focusing on the imaging vulnerability of atherosclerotic plaques, thrombolytic resistance, real-time cerebral perfusion, and penumbra.
Stroke is the major cause of death and the leading cause of disability worldwide,1 and the best treatment is prevention by controlling the risk factors for atherosclerosis, such as hypertension, diabetes, and hyperlipidemia.2 Atherosclerosis is characterized by progressive accumulation of lipids and inflammatory cells within the artery wall.3,4 It is a diffuse systemic disease;3 however, some atherosclerotic plaques are prone to rupture and thereby cause sudden thromboembolic vascular occlusion, while others are clinically silent.5-7 Therefore, to prevent stroke, clinicians need to localize high-risk vulnerable plaques, which has been a great challenge to date.
If prevention fails and ischemic stroke occurs, some patients can be treated with thrombolytic drugs that dissolve blood clots obstructing blood flow to the brain.8-10 The likelihood of recovery with little or no disability is at least 30% higher in treated patients than in those who do not receive the drugs in time.11 However, thrombolytic treatment in itself can cause the complication of intracranial hemorrhage, and it is this complication that hampers the wider application of thrombolytic therapy.12-14 Identifying predictors for the occurrence of thrombolysis-induced symptomatic intracranial hemorrhage is a very important objective that would make these therapies safer.15,16 In a similar context, predicting thrombolytic resistance in an individual patient would allow more effective stratification of treatments.
If thrombolysis fails or hemorrhagic complication occurs, decompressive surgery-including hemicraniectomy and durotomy with temporal lobe resection-remains the most attractive option for treating ischemic brain swelling.17-19 Additional surgery, "strokectomy" of parts of the frontal or temporal lobe, may be needed in younger patients who fail to improve.20 Currently, surgeons do not have sophisticated intra-operative guiding systems to monitor and help control cerebral perfusion, allowing them to distinguish and delineate "riskier but salvageable" cerebral areas from irrevocably infarcted sites during these procedures.
Unfortunately, studies based on conventional structural imaging tools such as magnetic resonance imaging (MRI) and computed tomography (CT) have had limited success in solving the above-described problems. Molecular imaging can visualize pathophysiologic alterations and promises to augment and supplement anatomy-based imaging.21-23 This brief review focuses on optical molecular imaging and its potential roles in the future of stroke management.
Molecular imaging is defined as the in vivo measurement of biological processes at the cellular and molecular levels.24-26 Molecular-imaging-based visualization of in vivo pathophysiologic processes could provide information regarding specific molecular alterations underlying the disease status of individual patients in real time.27-29 By providing molecular information unobtainable using conventional anatomy-based imaging modalities, molecular imaging would allow 1) earlier detection of diseases, 2) precise discrimination of the stable versus unstable disease status, and 3) both diagnostic and therapeutic quantitative monitoring of disease progression.30-32 The various modalities of molecular imaging (CT/MRI/fluorescent optical imaging/positron-emission tomography, PET/single-photon-emission CT/SPECT/ultrasound) have their own specific advantages and disadvantages that are related to how the images are generated.21,32
The application of fluorescent proteins or fluorochromes to the life sciences during the last 2 decades has considerably advanced basic and translational biological research, and these advances are now beginning to affect clinical practice.29,33,34 Fluorescence involves the absorption of light at characteristic wavelengths, and the emission of the stored energy at longer wavelengths.35 The advantages of fluorescence as a molecular imaging modality include picomolar molecular sensitivity, absence of ionizing radiation, the possibility of using it in many modalities with different scales, and relatively low cost. However, poor tissue penetration capability is a major obstacle to overcome.36,37 The attenuation of light by tissue is lowest in the near-infrared (NIR, 700-900 nm) region, and imaging in this region offers 1) markedly less photon absorption by blood hemoglobin, lipid, and water, allowing light to penetrate centimeters into the body; and 2) substantially reduced tissue autofluorescence, enabling higher sensitivity detection of targeted NIR fluorescent (NIRF) molecular imaging agents against a low background.31,36,38 This technology can potentially be combined with a non-invasive optical tomography system, intra-operative NIRF imaging system, or fluorescence-sensing catheter-based system.39 Jaffer et al. recently demonstrated that a NIRF-sensing catheter based on a clinical coronary artery guidewire could detect in vivo cathepsin B (CatB) protease activity in rabbit vessels the size of human coronary arteries in real time.
NIRF imaging requires a much smaller dose of fluorescence probes to detect molecules of interest-nanomoles of fluorochromes can be detected, compared to micromoles for MRI or millimoles for CT.31,32 The relatively high spatial resolution (typically less than 1 mm) of the catheter-based imaging system is another advantage of fluorescence imaging.39 Scatter reduces the spatial resolution of non-invasive fluorescence molecular tomography (FMT) relative to using an endoscopic imaging device (-1 mm), and is within the range of resolution provided by SPECT and PET.36
Studies have demonstrated that the formation of a thrombus due to rupture of unstable atherosclerotic plaques, followed by thrombotic or embolic occlusion of an artery, is the leading cause of stroke, accounting for up to 80% of cases of ischemic stroke in some autopsy series.10,40 There is a pressing need for tools to identify these vulnerable plaques, and thereby identify patients and lesions at high risk for vascular events, so that risk-altering treatments might be offered to improve clinical outcomes. According to the current practice guidelines and consensus, a carotid lesion is likely to cause ischemic stroke when stenosis of over 60 or 70% is detected by angiography.41 However, it has become clear that many strokes are attributable to plaques in the arteries with stenosis of 50% or less, highlighting the importance of plaque ruptures as a causative mechanism.42-44 Rupture-prone vulnerable plaques are not well identified by conventional measures of stenosis.45-47 Ultrasonic characterization of plaques as heterogeneous or of low echodensity on carotid duplex ultrasonography has been regarded by some as suitable for detecting unstable plaques, but firm conclusions await further studies.48,49 Plaque size and morphology (Fig. 1) are poor substitutes for the molecular events that shaped them, and the measurement of underlying molecular states provides the best hope for determining the propensity to cause complications.
For asymptomatic patients with significant carotid stenosis, the number needed to treat to prevent one stroke from any cause at two years is 67, which could increase to 111 to prevent one large-artery stroke at 2 years, since not all strokes originate from a narrowed internal carotid artery.50 For symptomatic patients, endarterectomy is beneficial in the long term even in the presence of a contralateral occlusion and increased perioperative risk.51,52 However, from a patient's standpoint, opting for a procedure with a 3-6% immediate perioperative risk to reduce a future stroke chance by 6-15% at 5 years in both asymptomatic and symptomatic cases can be daunting.53 This illustrates the difficulty of weighing the risks and benefits in treating an individual patient.51
Characteristic features of vulnerable atherosclerotic plaques are accumulation of inflammatory cells inside the plaque (Fig. 2), formation of a large lipid core, and thinning of the overlying fibrous cap.5 Heavily clustered macrophages in and around the shoulder region of the cap would secrete matrix-disorganizing proteolytic enzymes such as cathepsins and matrix metalloproteinases (MMPs), which could render atherosclerotic plaques prone to rupture.6,54 Thus, inflammatory protease activity could be regarded as a hallmark of the plaque vulnerability. In fact, using the Affymetrix gene chip to profile genes expressed in stable and unstable atherosclerotic plaques revealed that the expressions of CatB, cathepsin S, and MMP-9 were up-regulated in unstable atherosclerotic plaques.55 Taken together, these data suggest that imaging protease activity in vivo could allow identification of vulnerable plaques.
The Weissleder group and others have developed protease-sensing NIRF molecular imaging agents.56-60 Unlike "always-on" probes such as nonspecific NIRF probes [e.g., indocyanine green (ICG)] or targeted NIRF probes relying on affinity ligands, the protease-sensing NIRF probes are "optically silent at injection" because of intra-molecular autoquenching between closely spaced fluorochromes. Enzyme-specific protease-mediated cleavage spatially separates fluorochromes so that they become dequenched and brightly fluorescent. Multiple NIR fluorochromes positioned on a polylysine backbone can be activated by CatB.34 The gelatinase substrate sequence GGPRQITAG has been used to synthesize a NIRF probe that can be activated by MMP-2/9.58 Animal studies using these activatable probes have shown that in vivo and ex vivo NIRF imaging could visualize the protease activity in the aortic or carotid atheromata of ApoE knock-out mice (Figs. 3 and 4).58,59,61,62 Immunohistochemical analyses demonstrated that the NIRF signal precisely reflected the spatial distribution of the inflammatory protease activities. In addition, the protease immunoreactivity co-localized with Mac-3 or CD-68 positive macrophages.
Despite the high expectations for and rapidly increasing interest in molecular imaging techniques, there are significant challenges in translating the advances from the laboratory to the atherosclerosis clinic. As a step toward clinical translation, we recently demonstrated that CatB NIRF imaging could reliably reflect the anti-atherosclerotic effect of atorvastatin and glucosamine as well as the pro-atherosclerotic effect of a high cholesterol diet (Fig. 4). We also revealed that plaque populations were heterogeneous within individuals, with some plaques showing high and others lower CatB-related signals.61 Notably, these differences in signal intensity could not be predicted by visual inspection of the plaques. In every case where imaging predicted an inflammatory component in the plaque, histological studies confirmed it to be the case.61 Regarding translation steps from animals to humans, catheter-based fluorescence imaging systems are likely to be available in the atherosclerosis clinic. In addition, it should be remembered that since NIR photons can potentially penetrate >5 centimeters into the body, noninvasive FMT systems may eventually detectNIRF signals from human carotid atheromata.36,37 Moreover, the protease-sensing probe is expected to enter clinical trials in 2010.31 Other molecular imaging biomarkers that reflect plaque vulnerability include annexin for apoptosis,63,64 integrins for angiogenesis,65 and inflammatory cells such as macrophages.66-68
To summarize, molecular optical imaging could contribute to stroke prevention by detecting protease activity in atherosclerotic plaques in vivo; it would thereby serve as a powerful tool for evaluating vascular inflammation, for determining individualized therapeutic strategies including pre-operative planning, and for monitoring the effects of therapeutic interventions.
Fibrinolytic therapy, which is the only FDA-approved treatment for acute cerebral infarction, inevitably imposes a hemorrhagic risk.10,13,14 Delayed or intra-arterial thrombolysis is associated with higher complication rates, and enormous effort has been expended to empirically define the risks and benefits of thrombolysis at different times after vascular occlusion.14 Predicting the thrombolytic resistance and hemorrhagic risk in individual patients would be extremely helpful when deciding whether or not to perform thrombolytic therapy in individual patients.
As a first step toward imaging thrombolytic resistance in vivo, we and others chose to devise and characterize a molecular imaging agent to probe for the activity of factor XIII (FXIII) enzyme, a thrombin activated transglutaminase.69-71 The FXIII probe contains NIR fluorochromes attached to the peptide sequence NQEQVSPLTLLK, which is specifically recognized by the coagulation enzyme as its substrate. The normal function of activated FXIII (FXIIIa) enzyme is to stabilize a thrombus by cross-linking fibrin fibers-thus increasing the tensile strength-so as to covalently bind molecules that impede plasmin activity, such as α2-antiplasmin.72
Imaging this coagulation enzyme activity may be useful to staging the thrombotic disease when determining the appropriate therapy by clarifying the acute-versus-chronic state of the clot, which is difficult or impossible to achieve with anatomic imaging only. When the probe was injected intravenously into mice with ferric-chloride-induced thrombi in the femoral artery, the FXIIIa-related NIRF signal intensity was proportional to the age of the thrombi.71
The activity of FXIIIa enzyme is expected to change far more rapidly than the clot volume, and may serve as an early predictor of the success or failure of a therapeutic regimen.73 In this context, we investigated whether FXIIIa imaging could reflect treatment effects. To study the effect of heparin in a mouse model of cerebral venous thrombosis, heparin was administered immediately before thrombus induction and the FXIII probe was injected 3 hours later.70 The FXIIIa-related NIRF signal, captured through a cranial window using an intravital fluorescence microscope (Fig. 5), was significantly weaker in the heparin-pretreated group than in the untreated group. Notably, the therapeutic response to the anticoagulation treatment could be monitored in real time. We are currently studying if FXIIIa imaging can predict thrombolytic resistance in an arterial stroke model. Thrombolytic resistance is likely multifactorial in origin, and hence other imaging or plasma biomarkers of thrombolytic resistance merit further investigations, such as plasminogen activator inhibitor-1, α2-antiplasmin, and factor V.74,75
The future combination of molecular optical imaging with fluorescence-sensing intra-arterial catheter systems is expected to be suitable for clinical application, with molecular diagnosis guiding therapy with fibrinolytic agents or thrombin inhibitors and their successor drugs in acute ischemic stroke. This approach may allow discrimination of thrombi that would be resistant to chemical thrombolytic agents. Early triage to mechanical recanalization and clot retrieval methods would be rational when chemical thrombolysis is expected to fail, if patients could be triaged appropriately to these treatments (Fig. 6).
Intra-operative molecular imaging may assist neurosurgeons who are performing procedures such as post-stroke decompression surgery or revascularization operations to visualize cerebral perfusion and penumbra in real time. Woitzik and colleagues76 introduced intraoperative cerebral videoangiography, an imaging system integrated into the surgical microscope, which allowed 1) real-time imaging of the cortical blood flow in patients undergoing decompressive craniectomy and 2) cortical perfusion areas to be stratified into ischemic core and penumbral areas. They used ICG, which binds tightly to plasma proteins and becomes confined to the vascular system; its short half-life and emission at a NIR wavelength (835 nm) make it an advantageous contrast agent for in vivo angiography. A cerebral blood flow index (the ratio of the difference in fluorescence intensity and rise time) was calculated to determine flow maps that were used to assign a significant portion of tissue (15-37%) to the penumbra,76 which is a potential target to maximize benefits from the decompressive craniectomy, which in turn reduces intracranial pressure and improves cerebral blood flow.77
Clinically, in vivo retinal angiography with ICG has long been used to visualize neovascularization and hemorrhage in retinal and choroidal tissues.78 In a mouse model with a cranial window, we showed that cerebral ICG-based angiography could reveal permeability of the blood-brain barrier by sensing ICG leakage from cerebral vessels due to thrombosis-induced venous hypertension in real time in vivo.70 The technique was recently used to evaluate cortical microvascularization to compensate for a reduced cerebral blood flow in Moyamoya disease.79 ICG-based angiography demonstrated that the microvascular density and diameter were significantly elevated in patients with Moyamoya disease.
In addition to the cerebral perfusion status, the penumbral area could be defined by various physiological parameters including decreased pH due to increased intracellular lactate accumulation.80-82 pH-Activatable molecular optical imaging probes conjugated to a cancer-targeting monoclonal antibody were developed recently.83 The probes consist of a boron-dipyrromethene (BODIPY) as a fluorophore and a dialkylated aniline that renders the fluorophore fluorescent at different acidic pHs. BODIPY dye with a nonprotonated form of dialkylated aniline is nonfluorescent, whilst the dye with a protonated form of aniline is highly fluorescent. Conjugation of the probe to a cancer-specific monoclonal antibody, trastuzumab, leads to recognition and internalization by HER2-positive cancer cells via the endosomal-lysosomal degradation pathway. An acidic pH in the lysosome then turns on the fluorophore, and hence the probe offers tumor specificity with a minimal background signal. It should be noted that the imaging agent is not an "always-on" probe like conventional targeted probes. Instead, it becomes fluorescent under specific conditions, which improves the specificity and sensitivity as in the case of protease activatable probes. More importantly, since the proton pump is required to maintain the acidic condition in the lysosome, only viable cancer cells can be visualized by the probe. This kind of pH-sensing probe may be useful in delineating cerebral penumbra and infarcted tissues.
Molecular imaging researchers are actively pursuing 1) the development of novel molecular imaging strategies, particularly multi-modal molecular imaging, 2) innovations in molecular imaging-based theranostics, and 3) bench-to-bedside translation of associated cutting-edge technologies. Cardiovascular and neurovascular molecular optical imaging researchers pursue similar aims. Multi-modal molecular imaging probes are detectable by optical, MRI, and nuclear approaches simultaneously, which provides both high molecular sensitivity and high anatomical resolution as shown by Kircher et al.84 combined MR and optical imaging. Nahrendorf et al. labeled dextranated and DTPA-modified magnetofluorescent nanoparticles with 64Cu to yield a macrophage-targeted trimodality reporter for combined MRI, PET, and optical imaging.67
Combining diagnostic molecular imaging with therapeutic (theranostic) approaches would allow diseases to be detected at an early stage, drug delivery to desired targets to be monitored, quantification of the therapeutic efficacy in an individual patient in vivo, and the treatment for the patient to be tailored based on the imaging results. The development and utilization of protease-mediated photodynamic agents could be regarded as a theranostic approach.85 The agents are nontoxic in their native state, but they become fluorescent and produce singlet oxygen on protease conversion; after being delivered and activated in and around the macrophages infiltrating vulnerable plaques, intravenously injected theranostic agents are expected to not only visualize rupture-prone plaques but also stabilize them as a photodynamic therapy while avoiding systemic phototoxicity.
Healthcare in the 21st century is evolving to be personalized, predictive, and preventive. Molecular imaging provides a fundamentally new type of information that allows a timely and precise assessment of pathophysiologic states at the cellular and molecular levels, thereby complementing that available from anatomically based imaging modalities. Molecular optical imaging will provide the information needed to provide personalized stroke care. Most importantly, well-designed translational animal studies and human trials will be key to establishing the use of molecular imaging tools in clinical stroke management.86
Figures and Tables
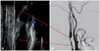 | Fig. 1Duplex ultrasonography and angiography imaging provide structural information about carotid atherosclerosis. In the carotid sonograph (A), the atherosclerotic plaque is shown to have heterogeneous echodensity (blue arrows). The digital subtraction angiogram (B) shows significant atherosclerotic narrowing of the internal carotid artery. Current stroke practice relies heavily on the degree of carotid stenosis, as demonstrated by these anatomy-based structural imaging modalities. Adapted from Kim and Jeong87 with permission from the Korean Medical Association. |
 | Fig. 2Three-dimensional carotid positron-emission tomography and computed tomography angiography imaging. 18Fluoro-deoxyglucose uptake is elevated in the right carotid artery (blue arrow), reflecting increased metabolism due to heavy infiltration of inflammatory cells such as macrophages in the atherosclerotic plaque. Adapted from Kim and Jeong87 with permission from the Korean Medical Association. The numbers on the pseudo-color bar indicate standard uptake values. |
 | Fig. 3Protease-activatable near-infrared fluorescent (NIRF) imaging to allow the visualization of vulnerable plaques in the aorta of a mouse. In the areas positive for cathepsin K (CatK) immunohistochemical staining (A, ×20, red-colored regions), the fluorescence activation of the intravenously-injected imaging agent59 by the CatK cleavage activity is evident in NIRF microscopy imaging of cryosections of the aortic atheromata in an ApoEknock-out mouse fed on a Western diet (B, ×20); the co-localization is illustrated by the yellow arrowheads. Higher magnification (C, ×40) reveals the derangement of undulated elastic fibers (red arrow), suggesting matrix disorganization. A stable portion of the artery without CatK activity or elastin degradation is indicated by the blue arrow. Taken together, these results suggest that molecular optical imaging can reflect matrix-disorganizing protease activity, allowing identification of rupture-prone inflammatory plaques. |
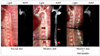 | Fig. 4Light imaging and protease-sensing molecular optical imaging to reflect the pro-atherosclerotic and anti-atherosclerotic effects of a high cholesterol diet and atorvastatin treatment, respectively. Eight-week-old ApoE knock-out mice were put on various diets: 1) a normal chow diet (left two panels), 2) a Western diet (center two panels), and 3) a Western diet with atorvastatin for 14 weeks (right two panels). The cathepsin B (CatB) NIRF imaging shows that the CatB-related signal is stronger in the mouse on a high cholesterol diet than in the mouse on a normal diet. In the animal on a Western diet with atorvastatin, the CatB-related signal is as low as that in the animal on a normal diet. Note that plaques that appear similar in the color photograph (of the animal on a Western diet) have different signal intensities in the CatB image (arrows and arrowheads). Adapted from Kim et al.88 with permission from the Korean Neurological Association. NIRF: near-infrared fluorescent. |
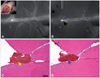 | Fig. 5
In vivo factor-XIII (FXIII) imaging to localize venous cerebral thrombi in a mouse. A mouse with cerebral venous th rombosis and a cranial window (inset) received a fluorescent imaging agent (A15) that could visualize the activity of the FXIII coagulation enzyme. Intravital microscopy imaging showed that the imaging agent initially remained intravascular, producing a cerebral angiogram (A). The NIRF signal subsequently decreased in all but thrombosed areas (B; image captured at 30 minutes after A15 injection). The representative H&E sections (C and D; acquired by cutting along the yellow and blue lines of B, respectively) of the superficial brain at the location of the frontal tip of the thrombus signal (black arrow in B) show the corresponding thrombus (yellow and blue arrows, respectively). In addition to the main body of the thrombus, a smaller thrombus in the adjacent vessel (asterisk in D) exhibits an FXIII-related NIRF signal (asterisk in B). Modified from Kim et al.70 with permission from the Korean Neurological Association. Scale bars: A, 1 mm; B, 150 µm. NIRF: near-infrared fluorescent. |
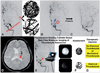 | Fig. 6Potential role of molecular optical imaging to guide thrombolytic therapy in acute stroke patients. Currently there is no established clinical tool for characterizing the resistance of a thrombus to thrombolytic drugs. One of the key determinants of thrombolytic resistance is fibrin cross-linking resulting from the activity of FXIII. FXIII activity or the density of cross-linked fibrin mesh cannot be measured in vivo (A). One patient (who was later found to have had a highly cross-linked clot) had received a urokinase infusion in the setting of acute stroke. It took a long time to lyse the thrombus occluding (red circle in B) the left middle cerebral artery (MCA). This resistant thrombus was associated with not only delayed recanalization but also with high doses of thrombolytic drug delivered into MCA perforating arteries or collateral arteries (blue arrows in B and C). The patient exhibited post-thrombolysis hemorrhage in the basal ganglia and corona radiata (red arrow in D). In the near future, the thrombolytic resistance of a thrombus is likely to become measurable in vivo and in real time using a fluorescence-sensing fiber-optic catheter and molecular imaging probes to characterize the nature of the thrombus (E). This could be of great benefit in triaging patients to appropriate treatments (F and G). |
Acknowledgements
This work was supported by a grant (A084274 to Dr. Dong-Eog Kim) from the Korean Ministry for Health, Welfare and Family Affairs.
References
1. Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009. 119:480–486.
2. Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006. 37:1923–1932.
5. Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc Pathol. 2004. 13:125–138.
6. Naghavi M, Falk E, Hecht HS, Jamieson MJ, Kaul S, Berman D, et al. From vulnerable plaque to vulnerable patient-Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol. 2006. 98(2A):2H–15H.

7. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000. 20:1262–1275.
8. Fibrinolytic Therapy Trialists' (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet. 1994. 343:311–322.
9. Bang OY. Multimodal MRI for ischemic stroke: from acute therapy to preventive strategies. J Clin Neurol. 2009. 5:107–119.

11. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995. 333:1581–1587.
12. The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997. 28:2109–2118.
13. Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies. Stroke. 2005. 36:2311–2320.

14. Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004. 35:11 Suppl 1. 2726–2730.

15. Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006. 12:1278–1285.

16. Rosell A, Foerch C, Murata Y, Lo EH. Mechanisms and markers for hemorrhagic transformation after stroke. Acta Neurochir Suppl. 2008. 105:173–178.

17. Diedler J, Sykora M, Blatow M, Jüttler E, Unterberg A, Hacke W. Decompressive surgery for severe brain edema. J Intensive Care Med. 2009. 24:168–178.

18. Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB, et al. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009. 8:326–333.

19. Köhrmann M, Schwab S. Hemicraniectomy for malignant middle cerebral artery infarction. Curr Opin Crit Care. 2009. 15:125–130.

20. Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007. 38:1655–1711.

22. Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003. 17:545–580.

23. Wickline SA, Lanza GM. Nanotechnology for molecular imaging and targeted therapy. Circulation. 2003. 107:1092–1095.

28. Wong FC, Kim EE. A review of molecular imaging studies reaching the clinical stage. Eur J Radiol. 2009. 70:205–211.

29. Yang M, Jiang P, Hoffman RM. Whole-body subcellular multicolor imaging of tumor-host interaction and drug response in real time. Cancer Res. 2007. 67:5195–5200.

30. Choudhury RP, Fisher EA. Molecular imaging in atherosclerosis, thrombosis, and vascular inflammation. Arterioscler Thromb Vasc Biol. 2009. 29:983–991.

31. Jaffer FA, Libby P, Weissleder R. Optical and multimodality molecular imaging: insights into atherosclerosis. Arterioscler Thromb Vasc Biol. 2009. 29:1017–1024.
32. Rudd JH, Hyafil F, Fayad ZA. Inflammation imaging in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009. 29:1009–1016.

33. Schwille P, Haupts U, Maiti S, Webb WW. Molecular dynamics in living cells observed by fluorescence correlation spectroscopy with one- and two-photon excitation. Biophys J. 1999. 77:2251–2265.

34. Weissleder R, Tung CH, Mahmood U, Bogdanov A Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999. 17:375–378.

35. Terai T, Nagano T. Fluorescent probes for bioimaging applications. Curr Opin Chem Biol. 2008. 12:515–521.

36. Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005. 23:313–320.

37. Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003. 9:123–128.

38. Chang K, Jaffer F. Advances in fluorescence imaging of the cardiovascular system. J Nucl Cardiol. 2008. 15:417–428.

39. Funovics MA, Weissleder R, Mahmood U. Catheter-based in vivo imaging of enzyme activity and gene expression: feasibility study in mice. Radiology. 2004. 231:659–666.

40. Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003. 2:43–53.

41. Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998. 339:1415–1425.

42. Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997. 336:1276–1282.

43. Crouse JR 3rd, Craven TE, Hagaman AP, Bond MG. Association of coronary disease with segment-specific intimal-medial thickening of the extracranial carotid artery. Circulation. 1995. 92:1141–1147.

44. Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002. 15:439–446.

45. U-King-Im JM, Young V, Gillard JH. Carotid-artery imaging in the diagnosis and management of patients at risk of stroke. Lancet Neurol. 2009. 8:569–580.

46. Nighoghossian N, Derex L, Douek P. The vulnerable carotid artery plaque: current imaging methods and new perspectives. Stroke. 2005. 36:2764–2772.
47. Yuan C, Kerwin WS, Ferguson MS, Polissar N, Zhang S, Cai J, et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J Magn Reson Imaging. 2002. 15:62–67.

48. Lindner JR, Song J, Xu F, Klibanov AL, Singbartl K, Ley K, et al. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation. 2000. 102:2745–2750.

49. Weinberger J, Azhar S, Danisi F, Hayes R, Goldman M. A new noninvasive technique for imaging atherosclerotic plaque in the aortic arch of stroke patients by transcutaneous real-time B-mode ultrasonography: an initial report. Stroke. 1998. 29:673–676.

50. Inzitari D, Eliasziw M, Gates P, Sharpe BL, Chan RK, Meldrum HE, et al. North American Symptomatic Carotid Endarterectomy Trial Collaborators. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. N Engl J Med. 2000. 342:1693–1700.

51. Lanzino G, Rabinstein AA, Brown RD Jr. Treatment of carotid artery stenosis: medical therapy, surgery, or stenting? Mayo Clin Proc. 2009. 84:362–387. quiz 367-368.

52. Usman AA, Tang GL, Eskandari MK. Metaanalysis of procedural stroke and death among octogenarians: carotid stenting versus carotid endarterectomy. J Am Coll Surg. 2009. 208:1124–1131.

53. Rerkasem K, Rothwell PM. Temporal trends in the risks of stroke and death due to endarterectomy for symptomatic carotid stenosis: an updated systematic review. Eur J Vasc Endovasc Surg. 2009. 37:504–511.

54. Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006. 47:8 Suppl. C13–C18.

55. Papaspyridonos M, Smith A, Burnand KG, Taylor P, Padayachee S, Suckling KE, et al. Novel candidate genes in unstable areas of human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006. 26:1837–1844.

56. Lee S, Cha EJ, Park K, Lee SY, Hong JK, Sun IC, et al. A near-infrared-fluorescence-quenched gold-nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew Chem Int Ed Engl. 2008. 47:2804–2807.

57. Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007. 116:1052–1061.

58. Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, et al. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006. 114:55–62.
59. Chen J, Tung CH, Mahmood U, Ntziachristos V, Gyurko R, Fishman MC, et al. In vivo imaging of proteolytic activity in atherosclerosis. Circulation. 2002. 105:2766–2771.

60. Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007. 115:377–386.

61. Kim DE, Kim JY, Schellingerhout D, Shon SM, Jeong SW, Kim EJ, et al. Molecular imaging of cathepsin B proteolytic enzyme activity reflects the inflammatory component of atherosclerotic pathology and can quantitatively demonstrate the antiatherosclerotic therapeutic effects of atorvastatin and glucosamine. Mol Imaging. 2009. 8:291–301.

62. Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, et al. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007. 115:2292–2298.

63. Sarai M, Hartung D, Petrov A, Zhou J, Narula N, Hofstra L, et al. Broad and specific caspase inhibitor-induced acute repression of apoptosis in atherosclerotic lesions evaluated by radiolabeled annexin A5 imaging. J Am Coll Cardiol. 2007. 50:2305–2312.

64. Kietselaer BL, Reutelingsperger CP, Heidendal GA, Daemen MJ, Mess WH, Hofstra L, et al. Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. N Engl J Med. 2004. 350:1472–1473.

65. Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003. 108:2270–2274.

66. Trivedi RA, Mallawarachi C, U-King-Im JM, Graves MJ, Horsley J, Goddard MJ, et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006. 26:1601–1606.

67. Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008. 117:379–387.

68. Hyafil F, Cornily JC, Feig JE, Gordon R, Vucic E, Amirbekian V, et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007. 13:636–641.

69. Kim DE, Tsuji K, Kim YR, Mueller FJ, Eom HS, Snyder EY, et al. Neural stem cell transplant survival in brains of mice: assessing the effect of immunity and ischemia by using real-time bioluminescent imaging. Radiology. 2006. 241:822–830.

70. Kim DE, Schellingerhout D, Jaffer FA, Weissleder R, Tung CH. Near-infrared fluorescent imaging of cerebral thrombi and blood-brain barrier disruption in a mouse model of cerebral venous sinus thrombosis. J Cereb Blood Flow Metab. 2005. 25:226–233.

71. Jaffer FA, Tung CH, Wykrzykowska JJ, Ho NH, Houng AK, Reed GL, et al. Molecular imaging of factor XIIIa activity in thrombosis using a novel, near-infrared fluorescent contrast agent that covalently links to thrombi. Circulation. 2004. 110:170–176.

72. Muszbek L, Yee VC, Hevessy Z. Blood coagulation factor XIII: structure and function. Thromb Res. 1999. 94:271–305.
73. Robinson BR, Houng AK, Reed GL. Catalytic life of activated factor XIII in thrombi. Implications for fibrinolytic resistance and thrombus aging. Circulation. 2000. 102:1151–1157.
74. Zhu Y, Carmeliet P, Fay WP. Plasminogen activator inhibitor-1 is a major determinant of arterial thrombolysis resistance. Circulation. 1999. 99:3050–3055.

76. Woitzik J, Peña-Tapia PG, Schneider UC, Vajkoczy P, Thomé C. Cortical perfusion measurement by indocyanine-green videoangiography in patients undergoing hemicraniectomy for malignant stroke. Stroke. 2006. 37:1549–1551.

77. Coutinho JM, Majoie CB, Coert BA, Stam J. Decompressive hemicraniectomy in cerebral sinus thrombosis: consecutive case series and review of the literature. Stroke. 2009. 40:2233–2235.

79. Czabanka M, Peña-Tapia P, Schubert GA, Woitzik J, Vajkoczy P, Schmiedek P. Characterization of cortical microvascularization in adult moyamoya disease. Stroke. 2008. 39:1703–1709.

80. Pestalozza IF, Di Legge S, Calabresi M, Lenzi GL. Ischaemic penumbra: highlights. Clin Exp Hypertens. 2002. 24:517–529.

81. Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008. 14:497–500.

82. Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999. 22:391–397.

83. Urano Y, Asanuma D, Hama Y, Koyama Y, Barrett T, Kamiya M, et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med. 2009. 15:104–109.

84. Kircher MF, Mahmood U, King RS, Weissleder R, Josephson L. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 2003. 63:8122–8125.
85. Choi Y, Weissleder R, Tung CH. Selective antitumor effect of novel protease-mediated photodynamic agent. Cancer Res. 2006. 66:7225–7229.

86. Kim DE, Kim JY, Schellingerhout D, Kim EJ, Kim HK, Lee S, et al. Protease Imaging of Human Atheromata Captures Molecular Information of Atherosclerosis, Complementing Anatomic Imaging. Arterioscler Thromb Vasc Biol. 2010. 30:449–456.

88. Kim DE, Kim JY, Kim EJ, Jeong SW. Molecular optical imaging of cathepsin-B proteolytic enzyme activity to reflect atherosclerosis pathophysiology and anti-atherosclerotic therapeutic effect. J Korean Neurol Assoc. 2009. 27:36–41.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download