Abstract
Background and purpose
Several clinical studies have demonstrated that patients with essential tremor (ET) may have cognitive deficits; however, there are no published data regarding detailed neuropsychological assessments of ET without dementia. We therefore conducted a case-control study of cognitive function in patients with ET.
Methods
The cohort for this study comprised 34 consecutive patients with ET without dementia and 33 age-matched controls, all of who completed a dementia-screening questionnaire and underwent a detailed neuropsychological investigation.
Results
Severe impairments were observed in most domains for the ET group compared to the controls, including attention, part of language function, verbal memory, and frontal executive functions.
Conclusions
Our results support the finding that the subclinical cognitive deficits characterized by attention, verbal memory impairments, and executive dysfunction are a clinical feature of ET. In addition, our results also support the finding that age at examination and educational status are the most important risk factors associated with cognitive deficits in patients with ET.
Essential tremor (ET) is a late-life neurologic disease that is characterized by action tremor.1,2 There is growing evidence that this disease is a multiple-system disorder because of additional motor features (e.g., intention tremor and ataxia) and nonmotor features (mild cognitive deficits and personality changes).3,4 Several studies have demonstrated that patients with ET may also have mild cognitive impairments (MCIs), mainly in frontal executive function and memory, and cross-sectional studies have shown that these are associated with prevalent dementia.5-11 However, there are no published data regarding detailed neuropsychological assessments of patients with ET without dementia in Korea. We therefore conducted a case-control study of cognitive function in patients with ET without dementia.
The initial study cohort comprised 38 consecutive patients with ET who were examined at the Movement Disorder Clinic of Kangnam St. Mary's Hospital between January and December 2007. Four of these patients were excluded from the study after comprehensive neuropsychological testing revealed that they had mild dementia. Thus, a final total of 34 patients who had no history or symptoms of memory impairment or other cognitive disorders with functional impairment (as revealed by a dementia-screening questionnaire) were enrolled in this study. The evaluation procedure comprised a detailed medical history, a physical and neurological examination, a neuropsychological assessment, and brain magnetic resonance imaging. The patient's history of medical and neurological problems was obtained from the patients and family members, or from other caregivers. All patients were diagnosed as having either definite or probable ET based on National Institutes of Health diagnostic criteria,12 and all were tested for mutations of the FMR gene of fragile X tremor and ataxia syndrome. Patients were excluded from the study if 1) they had neurological abnormalities related to systemic or other neurological diseases, 2) they were taking medications reported to influence cognition, such as anticholinergics or beta-blocking agents, or 3) they were found to have an FMR gene mutation.
The age-matched controls (n=33) did not have a history or symptoms of tremor, memory impairment, or other cognitive dysfunctions, as revealed by a dementia screening questionnaire, and they had no history of other neurological diseases, such as head trauma, epilepsy, and stroke, or brain surgery. This study was approved by the local ethics committee, and each patient provided written informed consent to participate.
The patient's general cognitive status and severity of dementia were evaluated by the Korean version of Mini-Mental State Examination (K-MMSE), Clinical Dementia Rating (CDR), and the sum of box (SOB) of CDR.13-15 Several cognitive domains were assessed by conducting a detailed battery of neuropsychological tests. The tests included an attention and working memory test (forward digit span, backward digit span, and letter cancellation), a language and related-functions test (comprehension, repetition, Boston Naming Test, writing, calculation, finger naming, right-left orientation, body part identification, and praxis), a visuospatial function test (drawing an interlocking pentagons and Rey-Osterrieth Complex Figure Copy Test), a verbal memory test [registration, recall, recognition of three words, Hopkins Verbal Learning Test (HVLT), immediate recall, delayed recall, and recognition], a nonverbal (visual) memory test (immediate recall, delayed recall, and recognition of a Rey's complex figure), a frontal executive function test (motor impersistence, contrasting program, go-no-go test, fist-edge-palm, alternating hand movement, alternating square and triangle, and Luria Loop Test), the word fluency test [Controlled Oral Word Association Test (COWAT) animal, supermarket, and Korean letters], and the Stroop Color and Word Test (word reading and color reading).15-17
The statistical analyses were performed using SPSS, version 13.0. Statistical comparisons of some of the demographic data between the groups, and neuropsychological test results were performed using an independent-samples t-test. Other group differences (data for nominal variables) were assessed with the chi-square test. The relationships between the general mental functions and the age at examination, symptom duration, educational status, and Barthel index were analyzed using Spearman rank correlation coefficients. The level of statistical significance was set at p<0.05.
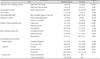
The demographic characteristics of the ET patients and controls are summarized in Table 1. The mean education level and age of the two groups were similar, however the gender distribution was not similar (26.5% and 54.5% of the ET and control groups, respectively, were men; p=0.026). The mean MMSE score was lower in the ET group than in the control group.
Table 2 and 3 present the characteristics of the ET and control groups as well as differences in the results of the detailed, comprehensive neuropsychological tests between the two groups. The impairments in most domains were more severe in the ET group than in the control group, including in attention and working memory, part of the language functions (Boston Naming Test), verbal memory (three-word recall and HVLT, free and delayed recall), and the frontal executive functions (the COWAT; p<0.01). The correlation analysis revealed that cognitive decline was significantly correlated with age and educational status, but not with either disease duration or Barthel index (Table 4).
Cognitive impairment is often associated with ET, although the deficits may be relatively subtle and not clinically apparent, or else they do not overtly affect the person's daily functioning. Executive dysfunction is thought to be at the heart of the cognitive dysfunction in ET patients and it is usually one of the earliest cognitive impairments found in these patients.5-11 Besides frontal executive dysfunction, previous studies have reported that most ET patients develop mild neuropsychological deficits across a range of cognitive functions (i.e., these deficits affect memory, visuospatial processing, and attention).10,11 However, these MCIs do not progress to full dementia in all ET patients, and the precise frequency and etiology of the dementia associated with ET are a matter of controversy. Nevertheless, early detection of the initial stages of dementia would have considerable clinical, therapeutic, and public health value.
In the present study we investigated the cognitive dysfunctions of ET without dementia and compared them with age-matched normal controls. As expected, the ET group had significantly more severe impairments of attention, verbal memory, and frontal executive functions compared to the normal control group. Many ET patients had cognitive decline ranging from normal to an MCI. In addition, the cognitive decline was significantly correlated with age and educational status, but not with disease duration or Barthel index.
The pathophysiologic basis for these findings is unclear, but there are several lines of evidence that ET is a neurodegenerative disease. ET is clinically progressive, with complex clinical characteristics accompanied by both cerebellar and extracerebellar signs, which may include olfactory deficits and cognitive dysfunction.5-11,18,19 Recent work has shown widespread white-matter pathology in ET, primarily involving the frontal area, but also with some temporoparietal area involvement.20 Other studies have shown that the cerebellum is functionally connected to the cerebral cortex through feedforward and feedback pathways.21,22 In addition, there are multiple lines of evidence that the circuitry involved includes the frontosubcortical pathways, which play a role in cognitive and affective processes in patients with ET.5-11 Conversely, these neuropsychological findings are similar to those observed in some cerebellar disorders.23,24 Taken together, the findings of previous studies and our own demonstrate that ET is associated with a poorer performance on tests of memory and frontal executive function. Our clinical findings therefore support the hypothesis that cognitive and motor fronto-cerebellar circuits are functionally associated in ET.
Our study does have several limitations that mean we are unable to resolve the pathophysiological association between cognitive and motor dysfunctions in ET. We did not test the impact of tremor severity on cognitive dysfunctions. Further studies using several motor parameters are needed to identify the pathologic basis for cognitive deficits in cases of ET. In addition, it is more helpful to perform functional metabolism studies and structural volumetric analyses to assess the associated functional pathogenesis of ET.
In summary, our results support the finding that the subclinical cognitive deficits characterized by attention and verbal memory impairments, and executive dysfunction are a clinical feature of ET. In addition, our results support the finding that age at examination and educational status are the most important risk factors associated with cognitive deficits in patients with ET, as with other types of dementia.25
Figures and Tables
Table 1
Demographic data and general cognitive functioning in patients with essential tremor and normal controls

Acknowledgments
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (project no. A060093).
References
1. Dogu O, Sevim S, Camdeviren H, Sasmaz T, Bugdayci R, Aral M, et al. Prevalence of essential tremor: door-to-door neurological exams in Mersin Province, Turkey. Neurology. 2003. 61:1804–1806.

2. Tanner CM, Goldman SM, Lyons KE, Aston DA, Tetrud JW, Welsh MD, et al. Essential tremor in twins: an assessment of genetic vs environmental determinants of etiology. Neurology. 2001. 57:1389–1391.

4. Lim ES, Seo MW, Woo SR, Jeong SY, Jeong SK. Relationship between essential tremor and cerebellar dysfunction according to age. J Clin Neurol. 2005. 1:76–80.

5. Tröster AI, Woods SP, Fields JA, Lyons KE, Pahwa R, Higginson CI, et al. Neuropsychological deficits in essential tremor: an expression of cerebello-thalamo-cortical pathophysiology? Eur J Neurol. 2002. 9:143–151.

6. Benito-León J, Louis ED, Bermejo-Pareja F. Neurological Disorders in Central Spain (NEDICES) Study Group. Population-based case-control study of cognitive function in essential tremor. Neurology. 2006. 66:69–74.

7. Gasparini M, Bonifati V, Fabrizio E, Fabbrini G, Brusa L, Lenzi GL, et al. Frontal lobe dysfunction in essential tremor: a preliminary study. J Neurol. 2001. 248:399–402.
8. Lacritz LH, Dewey R Jr, Giller C, Cullum CM. Cognitive functioning in individuals with "benign" essential tremor. J Int Neuropsychol Soc. 2002. 8:125–129.

9. Benito-León J, Louis ED, Bermejo-Pareja F. Neurological Disorders in Central Spain Strudy Group. Elderly-onset essential tremor is associated with dementia. Neurology. 2006. 66:1500–1505.

10. Lombardi WJ, Woolston DJ, Roberts JW, Gross RE. Cognitive deficits in patients with essential tremor. Neurology. 2001. 57:785–790.

11. Sahin HA, Terzi M, Ucak S, Yapici O, Basoglu T, Onar M. Frontal functions in young patients with essential tremor: a case comparison study. J Neuropsychiatry Clin Neurosci. 2006. 18:64–72.

12. Jankovic J. Essential tremor: clinical characteristics. Neurology. 2000. 54(11):Suppl 4. S21–S25.
13. Kang YW, Na DL, Hahn SH. A validity study on the Korean minimental state examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997. 15:300–308.
14. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Yoon SJ, et al. Estimating the validity of the Korean version of expanded clinical dementia rating (CDR) scale. J Korean Neurol Assoc. 2001. 19:585–591.
15. Kang YW. Samsung neuropsychological screening battery, Current research in dementia. 1998. Seoul: The Korean Dementia Association;99–107.
16. Kim H, Na DL. Korean-Boston Naming Test. 1997. Seoul: Hakji Co.
17. Kim E, Kim H, Na DL. Naming deficits in patients with dementia of the Alzheimer type: error analysis of Korean version-Boston Naming Test. J Korean Neurol Assoc. 1997. 15:1012–1021.
18. Louis ED, Bromley SM, Jurewicz EC, Watner D. Olfactory dysfunction in essential tremor: a deficit unrelated to disease duration or severity. Neurology. 2002. 59:1631–1633.

19. Applegate LM, Louis ED. Essential tremor: mild olfactory dysfunction in a cerebellar disorder. Parkinsonism Relat Disord. 2005. 11:399–402.

20. Shin DH, Han BS, Kim HS, Lee PH. Diffusion tensor imaging in patients with essential tremor. AJNR Am J Neuroradiol. 2008. 29:151–153.

22. Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000. 31:236–250.

23. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998. 121:561–579.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download