Abstract
Background
Acute ischemic stroke secondary to aortic dissection (AoD) is challenging in the era of thrombolysis owing to the diagnostic difficulty within a narrow time window and the high risk of complications.
Case Report
A 64-year-old woman with middle cerebral artery occlusion syndrome admitted to the emergency room within intravenous recombinant tissue plasminogen activator (rt-PA) time window. Her neurological symptoms improved during thrombolysis, but chest and abdominal pain developed. Repeated history-taking, physical examination, and imaging studies led to the timely diagnosis and surgical treatment of AoD, which produced a successful outcome.
Aortic dissection (AoD) is a rare cause of acute ischemic stroke. For acute ischemic stroke patients who are eligible for thrombolysis, AoD is particularly challenging owing to the difficulty of diagnosis within a narrow time window and the high risk of life-threatening complications for thrombolysis. We report a patient with acute ischemic stroke secondary to AoD who was treated with intravenous recombinant tissue plasminogen activator (rt-PA). Clinical suspicion and examination were invaluable for the timely diagnosis and treatment. We also review the literature to discuss the risks and benefits of thrombolytic therapy for these patients.
A 69-year-old woman was referred for hemiparesis. At 6:00 pm, she lost consciousness and was brought to a nearby hospital. She recovered consciousness after 30 minutes, but left-side hemiparesis was recognized. The results of brain CT were unremarkable, and she was transferred to our hospital for thrombolysis.
She arrived at our emergency room 110 minutes after the onset. She complained of transient chest discomfort. Concurrent acute myocardial infarction was suspected, but her electrocardiogram and cardiac enzymes were normal, and there was no cardiac murmur on the initial examination. The neurology stroke team was activated 125 minutes after the onset.
Her initial National Institutes of Health Stroke Scale (NIHSS) score was 6 due to the presence of a visual field defect, eyeball deviation, and left hemiparesis. Her blood pressure was 140/90 mmHg, and the glucose level and platelet count were normal. Review of the brain CT performed in the referring hospital revealed no abnormalities. She had no contraindications for intravenous rt-PA. During the evaluation, her NIHSS score increased to 8 owing to the progression of hemiparesis. Since a routine chest X-ray is not recommended as an initial evaluation for intravenous rt-PA in recent guidelines,1 this was not performed in order to avoid treatment delay before rt-PA treatment. Intravenous rt-PA was started 146 minutes after the onset. During treatment, we performed a chest X-ray and then CT angiography to investigate the occlusion of large vessels, for which combined intravenous and intra-arterial thrombolysis is considered in our protocol.
Her NIHSS score improved from 8 to 4 during the treatment, but chest and abdominal pain developed. CT angiography revealed no occlusion in the major intracranial arteries, but her extracranial carotid arteries could not be reconstructed, probably due to slow flow or a technical problem. However, bilateral carotid dissections were suspected on the source images (Fig. 1). Mediastinal widening was also suspected on the chest X-ray. A repeated physical examination revealed bilaterally diminished pedal pulses and a newly developed diastolic murmur. Repeated history-taking revealed chest pain, after which she lost consciousness.
We stopped intravenous rt-PA after infusing 60% of the total dose. An urgent chest CT revealed a Stanford type A AoD extending to the aortic valve. Emergent aortic surgery was started at 10:00 pm. Although the surgery took more than 10 hours due to the impaired hemostasis, she recovered without neurological deficit. Follow-up MRI performed 13 days postsurgery revealed small high signal intensity lesions on diffusion-weighted images, but the intracranial and extracranial arteries were patent (Fig. 2), and at 3 months her NIHSS score was 0.
AoD is a rare cause of ischemic stroke, but it is challenging in the era of thrombolysis owing to the diagnostic difficulty within a narrow time window and the high risk of life-threatening hemorrhagic complications. About 30% of patients with Stanford type A dissection show initial neurologic symptoms, but one-third of them do not report typical pain. More than half of the neurologic symptoms are attributable to acute ischemic stroke.1 As it is not practical to perform the diagnostic tests for AoD in every patient who is eligible for thrombolysis, clinical suspicion and examination are invaluable. In the present case, we should have paid attention to the loss of consciousness since this is not a usual presentation of middle cerebral artery occlusion. Examination of the peripheral pulse might have led to a faster diagnosis. Although a chest X-ray is not recommended as a routine initial evaluation before rt-PA administration in order to avoid treatment delay, it is recommended in patients with clinical or other evidence of acute cardiac or pulmonary disease,2 as in the present case. Our case also demonstrates the importance of patient monitoring during rt-PA treatment. Although a diagnosis was not made before rt-PA initiation, we were alert to her clinical changes. Repeated history-taking, physical examination, and careful interpretation of CT angiography led to a timely diagnosis and surgical treatment.
It is not clear whether rt-PA is indicated for acute ischemic stroke secondary to AoD, especially for patients expected to have severe disability without treatment. Current guidelines do not state specifically whether this situation is contraindicated for intravenous rt-PA. Surgeons are reluctant to perform surgery on AoD patients complicated by acute ischemic stroke. However, the outcome of AoD is usually poor without surgery.
Thrombolysis might contribute to dissection extension or rupture by interfering with thrombus formation at the intimal tear, increasing the risk of early death due to worsening hemothorax, or hemopericardium. Thrombolysis can also delay surgery and interfere with hemostasis. This issue has been a concern in the era of acute myocardial infarction because AoD can simulate acute myocardial infarction. The outcome of these patients after rt-PA treatment was poor, with a reported mortality of up to 71%.3 A literature review of the stroke field revealed three cases treated with thrombolysis,1,4,5 and seven cases given only a loading dose or considered but not treated,6-12 as summarized in Table 1; the mortality rates were 75% and 43% in the former and latter cases, respectively. Among the treated patients, one death was attributed to cardiac tamponade and intrapleural hemorrhage considered to be related to thrombolysis. In the present case, thrombolysis could contribute to dissection extension considering the presence of recurrent dissecting pain and the newly developed aortic regurgitation murmur after treatment. Among the patients considered but not treated or given only a loading dose, two out of the three deaths were caused by stroke and presumed hypoxic brain damage. The outcomes of the survivors given only a loading dose or considered but not treated were relatively favorable considering their initial stroke severity (Table 1).
The efficacy of thrombolysis should be considered in the light of the stroke mechanism in AoD. The most common mechanism is mechanical obstruction of the common carotid arteries due to the dissection extending to the supra-aortic area.1 Complete occlusion might be resistant to thrombolysis because of limited rt-PA delivery to the occlusion site or rt-PA not being effective for mechanical occlusion by the intimal flap. For partial or transient occlusion, spontaneous reperfusion might be possible without applying a high-risk treatment. Neurological symptoms were transient in many cases.1,6,7,10,11 In our patient the partial obstruction in both carotid arteries was confirmed during surgery. However, there is no extension of dissection to the supra-aortic area in onethird of patients with stroke, in which thromboembolism and/or severe hypotension are considered as possible mechanisms.1 Therefore, thrombolysis for ischemic stroke secondary to AoD might be efficacious in the minority of patients with a thromboembolic mechanism.
In conclusion, allowing for the known risks and benefits, intravenous rt-PA might not be indicated in most patients with acute ischemic stroke secondary to aortic dissection. Clinical suspicion and examination are invaluable for diagnosing AoD as an etiology of acute ischemic stroke, especially when considering the treatment of thrombolysis.
Figures and Tables
 | Fig. 1CT angiography revealed no occlusion in the intracranial major arteries (A), but suggested dissection in the bilateral extracranial carotid arteries on the source images (B, black arrows). Emergent chest CT showed a Stanford type A aortic dissection (C, white arrows). |
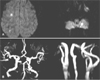 | Fig. 2Follow-up MRI performed 13 days postsurgery revealed small infarctions (A), but no occlusions in the extracranial and intracranial arteries (B). |
References
1. Gaul C, Dietrich W, Friedrich I, Sirch J, Erbguth FJ. Neurological symptoms in type A aortic dissections. Stroke. 2007. 38:292–297.

2. Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007. 38:1655–1711.

3. Kamp TJ, Goldschmidt-Clermont PJ, Brinker JA, Resar JR. Myocardial infarction, aortic dissection, and thrombolytic therapy. Am Heart J. 1994. 128:1234–1237.

4. Fessler AJ, Alberts MJ. Stroke treatment with tissue plasminogen activator in the setting of aortic dissection. Neurology. 2000. 54:1010.

5. Ibaraki T, Fukumoto H, Nishimoto Y, Nishimoto M, Suzuki S, Morita H. [Surgical management of acute type A aortic dissection with a complaint of disturbance of consciousness; report of a case]. Kyobu Geka. 2002. 55:1053–1056.
6. Chua CH, Lien LM, Lin CH, Hung CR. Emergency surgical intervention in a patient with delayed diagnosis of aortic dissection presenting with acute ischemic stroke and undergoing thrombolytic therapy. J Thorac Cardiovasc Surg. 2005. 130:1222–1224.

7. Uchino K, Estrera A, Calleja S, Alexandrov AV, Garami Z. Aortic dissection presenting as an acute ischemic stroke for thrombolysis. J Neuroimaging. 2005. 15:281–283.

8. Flemming KD, Brown RD Jr. Acute cerebral infarction caused by aortic dissection: caution in the thrombolytic era. Stroke. 1999. 30:477–478.

9. Villa A, Molgora M, Licari S, Omboni E. Acute ischemic stroke, aortic dissection, and thrombolytic therapy. Am J Emerg Med. 2003. 21:159–160.

10. Wright V, Horvath R, Baird AE. Aortic dissection presenting as acute ischemic stroke. Neurology. 2003. 61:581–582.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download