Abstract
Background and Purpose
Mutations of the skeletal muscle sodium channel gene SCN4A, which is located on chromosome 17q23-25, are associated with various neuromuscular disorders that are labeled collectively as skeletal muscle sodium channelopathy. These disorders include hyperkalemic periodic paralysis (HYPP), hypokalemic periodic paralysis, paramyotonia congenita (PMC), potassium-aggravated myotonia, and congenital myasthenic syndrome. This study analyzed the clinical and mutational spectra of skeletal muscle sodium channelopathy in Korean subjects.
Methods
Six unrelated Korean patients with periodic paralysis or nondystrophic myotonia associated with SCN4A mutations were included in the study. For the mutational analysis of SCN4A, we performed a full sequence analysis of the gene using the patients' DNA. We also analyzed the patients' clinical history, physical findings, laboratory tests, and responses to treatment.
Results
We identified four different mutations (one of which was novel) in all of the patients examined. The novel heterozygous missense mutation, p.R225W, was found in one patient with mild nonpainful myotonia. Our patients exhibited various clinical phenotypes: pure myotonia in four, and PMC in one, and HYPP in one. The four patients with pure myotonia were initially diagnosed as having myotonia congenita (MC), but a previous analysis revealed no CLCN1 mutation.
The skeletal muscle sodium channel comprises a principal pore-forming and voltage-sensing subunit (the alpha subunit), which is associated with an accessory beta-1 subunit. Its alpha subunit is encoded by the gene SCN4A, which is located on chromosome 17q23-25,1 comprises 24 exons with a 5.5-kb open reading frame,2 and is associated with various neuromuscular disorders. The beta-1 subunit has not been reported to be linked to any human disease.
More than 40 different types of SCN4A mutation have been identified, most of which are single-base substitutions producing missense mutations.3 In contrast to the chloride and calcium channelopathies, which produce relatively uniform phenotypes, SCN4A mutations produce several clinically distinct skeletal muscle disorders including hyperkalemic periodic paralysis (HYPP), paramyotonia congenita (PMC), potassium-aggravated myotonia (PAM), hypokalemic periodic paralysis, and congenital myasthenic syndrome.4-8 Previous studies have also shown that certain SCN4A mutations are associated with a specific phenotype, and it has been suggested that the mutation-specific differences in aberrant channel gating behavior underlies the genotype-phenotype correlation in skeletal muscle sodium channelopathy.9
In this context, we studied six Korean patients with SCN4A mutations who showed various clinical types of nondystrophic myotonia and periodic paralysis syndromes. We reasoned that this would help to clarify the genotype-phenotype correlations in Korean patients with this group of disorders. We therefore performed a mutational analysis of SCN4A in these patients, and analyzed their clinical features in detail.
Six unrelated Korean patients with nondystrophic myotonia and periodic paralysis syndrome were included in the study. The patients comprised one patient with HYPP, one with PMC, and four with pure myotonia. Mutation of CLCN1 had previously been excluded in all four patients with the pure myotonia phenotype, by full sequence analysis of its coding regions.10 The control group comprised 100 healthy Koreans. All of the patients and controls provided written informed consent to participate in this study, which was reviewed and approved by the Pusan National University Hospital Institutional Review Board.
A detailed clinical history was taken from all patients, and all underwent a standard neurological examination. History-taking revealed several clinical data, including age of onset, first clinical symptom, the most disabling symptom at the time of presentation, and family history. On neurological examination, special notice was taken of the presence of muscle atrophy or hypertrophy, distribution of muscle weakness (if any), status of the deep-tendon reflexes, and types of maneuver that provoke myotonia. All patients submitted to electrodiagnostic studies, including routine nerve conduction studies and needle electromyography (EMG) from at least two different muscles in each extremity. Routine laboratory tests were performed, including complete blood count, liver and renal function tests, thyroid function tests, blood glucose, electrolytes, serum creatine kinase levels, chest X-ray, and electrocardiogram. Muscle biopsy procedures were performed in three patients. The biopsied muscle samples were flash frozen in isopentane prechilled with liquid nitrogen and then processed for routine histochemical reactions including hematoxylin-eosin, modified Gomori trichrome, NADH, and ATPase stains.
The SCN4A mutations were identified by direct sequence analysis of whole coding regions of the gene. DNA was extracted from the patients' anticoagulated whole blood, and 21 sequence specific primer pairs covering the entire coding region of SCN4A were subjected to PCR amplification. Primer sequence data and information on the PCR conditions are available upon request.
The amplified PCR products were separated on 2% agarose gels, purified, cycle-sequenced with PCR primers using the BigDyeTerminator Sequencing Kit (PE Applied Biosystems, Foster, CA, USA), and electrophoresed using an ABI PRISM 3730XL DNA analyzer (PE Applied Biosystems).
PCR-restriction fragment length polymorphism (RFLP) was conducted on patient 3 and the 100 normal controls to confirm the presence of the novel c.673C>T (p.R225W) mutation found in this patient and to establish that it is not a benign polymorphism. The mutation was identified using the restriction enzyme Aci I to discriminate the mutated allele from normal alleles. The primer pair 5'-TGCACTGTCCT TCCCAACCC-3' (forward) and 5'-GCCTCTCAAACGCC CATCCT-3' (reverse) was used for the PCR reaction. Since the mutated allele will lose the normal Aci I restriction site, a heterozygous mutation of c.673C>T will produce three DNA bands at 472, 245, and 227 bp, while normal alleles will produce bands at only 245 and 227 bp. All enzyme digestion reactions were conducted at 37℃ for 2 hours, and the electrophoresis was performed on 2% agarose gel.
The sequence analysis revealed four different mutations including one that has not been described previously. The types and locations of the identified mutations are summarized in Figs. 1 and 2, and Table 1. A novel missense mutation, c.673C>T, was identified in exon 5 from patient 3, which is expected to change a codon for arginine into tryptophan at position 225 (p.R225W). This is considered a pathogenic mutation because 1) we were unable to observe the same alteration in any of the 100 normal controls using PCR-RFLP analysis (Fig. 3A), and 2) the mutated amino acid arginine at position 225 is highly conserved between different species in multiple alignment analysis of the amino acid sequence (Fig. 3B).
The clinical features of the six patients are summarized in Table 2. They comprised six men with ages of onset from birth to 30 years. The four patients with the pure myotonia phenotype (patients 1, 2, 3, and 6) had myotonia with the warm-up phenomenon, which initially affected predominantly the lower extremities. In this group of patients, muscle stiffness was provoked by voluntary contraction of the skeletal muscles after a period of rest, and typical myotonic discharges were observed on EMG. Patient 4 presented with myotonia that worsened by repetitive exercise and cold exposure, and was classified as having the PMC phenotype. EMG in this patient also revealed prominent myotonic discharges. Periodic weakness was the main symptom in patient 5, together with elevated serum potassium levels. Needle EMG also revealed myotonic discharges.
We have identified four different SCN4A mutations (one of which has not been reported previously) in six unrelated Korean patients with nondystrophic myotonia and periodic paralysis syndrome. A novel heterozygous missense mutation c.673C>T (p.R225W) was found in patient 3, who presented with mild nonpainful pure myotonia (Table 1 and 2). This mutation is located at the cytoplasmic side of transmembrane S3 segment of domain I (DI/S3; Figs. 1 and 2), and is noteworthy because most of the previously reported SCN4A mutations are clustered in domains III or IV of the protein. Furthermore, it is the most proximal mutation ever reported in SCN4A. Thus far only two mutations affecting domain I have been reported.11,12 This mutation is also remarkable because there is only one previous report of an SCN4A mutation affecting the S3 segment (p.L1433R).13
Another mutation, c.2078T>C (p.I693T), was identified in patient 4, who exhibited a typical PMC phenotype. This mutation is located at the cytoplasmic link between S4 and S5 of DII, and has been reported previously in patients with PMC (Table 1, Figs. 1 and 2).14
The c.3466G>A (p.A1156T) mutation was shared by two of our patients who exhibited completely different phenotypes (Table 1, Figs. 1 and 2): while patient 2 had pure myotonia affecting the lower extremities, with warm-up phenomenon, patient 5 manifested as HYPP without clinical history or signs of myotonia. Although the mutation p.A1156T has been described previously both in patients with HYPP and PMC,15 it has never been associated with the pure myotonia phenotype, as in our patient 2. This phenotypic variability observed in individuals harboring the same SCN4A mutation strongly suggests that the genetic background-and perhaps other epigenetic factors-influences the clinical expression of particular mutations.
The c.3917G>A (p.G1306E) mutation, located at the cytoplasmic linker between domains III and IV (ID3-4), was also found in two patients, in both of whom it manifested as pure myotonia (patients 1 and 6). This mutation had been reported in patients with PAM and has been functionally characterized (Table 1, Figs. 1 and 2).16,17
Our study shows that SCN4A mutations produce different phenotypes of nondystrophic myotonia and periodic paralysis syndrome. We have also shown that certain SCN4A mutations may not be associated with specific clinical phenotypes, suggesting poor genotype-phenotype correlations in skeletal muscle sodium channelopathy.
In sodium channelopathy, there is a group of patients who manifest with pure myotonia. These patients exhibit one of several clinical phenotypes including myotonia fluctuans, myotonia permanens, and acetazolamide-responsive myotonia.18 They are distinct from those with PMC by the absence of paradoxical myotonia and cold sensitivity. Since many such patients have myotonia that becomes much more pronounced after the ingestion of potassium-rich food, the condition has been collectively called PAM. However, some patients with SCN4A-related pure myotonia do not exhibit such potassium sensitivity,11,19 and thus the condition may be more appropriately labeled sodium-channel myotonia. The myotonia in this group of patients is very similar to that observed in those with myotonia congenita (MC), and clinical differentiation may not be possible. The four patients with pure myotonia in our study had also initially been considered as having MC, but they were finally shown to have SCN4A mutations. A recent study involving the screening of SCN4A and CLCN1 mutations in large numbers of patients with nondystrophic myotonia found that 20% of patients who had been clinically diagnosed as MC had SCN4A mutations.20
Our study also showed that sodium channelopathy comprises highly variable clinical phenotypes, and that the genotype-phenotype correlation is not unequivocal. Although careful clinical evaluation is of great help, we suggest that a definite molecular diagnosis using sequence analysis of both SCN4A and CLCN1 is essential for the differential diagnosis of sodium channelopathy from other conditions, especially when a patient presents with pure myotonia.
Figures and Tables
Fig. 1
Membrane-folding model of the sodium channel alpha subunit and locations of the missense mutations identified in the present study. A novel mutation, p.Arg 225Trp, is located at the transmembrane S3 segment of domain I.
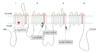
Fig. 2
Chromatograms of the patients. A: A novel mutation in exon 5 was identified in patient 3. A substitution of cytosine to thymidine at position 673 changes the codon for arginine at position 225 into tryptophan. B: A substitution of thymidine to cytosine at position 2078 in patient 4 changes the codon for isoleucine at position 693 into threonine. C: A substitution of guanine to adenine at position 3466 in patients 2 and 5 changes the codon for alanine at position 1156 into threonine. D: A substitution of guanine to adenine at position 3917 in patients 1 and 6 changes the codon for glycine at position 1306 into glutamate.

Fig. 3
A: The results of PCR-restriction fragment length polymorphism using restriction enzyme Aci I for the identification of the novel c.673C>T (p.Arg225Trp) mutation. No similar digestion pattern was observed among 100 normal control chromosomes. M, 100-bp molecular marker; U, undigested PCR product from patient 3; P, patient 3; numbers, control. B: Multiple alignment of the homologous sodium channel alpha subunit protein between different species of eukaryotes. The mutated protein in patient 3 (p.Arg 225Trp, arrowheads) is highly conserved between species.

Acknowledgements
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-331-E00201).
References
1. George AL Jr, Ledbetter DH, Kallen RG, Barchi RL. Assignment of a human skeletal muscle sodium channel alpha-subunit gene (SCN4A) to 17q23.1-25.3. Genomics. 1991. 9:555–556.

2. George AL Jr, Crackower MA, Abdalla JA, Hudson AJ, Ebers GC. Molecular basis of Thomsen's disease (autosomal dominant myotonia congenita). Nat Genet. 1993. 3:305–310.

3. Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NS, et al. Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat. 2003. 21:577–581.

4. Ptácek LJ, George AL Jr, Griggs RC, Tawil R, Kallen RG, Barchi RL, et al. Identification of a mutation in the gene causing hyperkalemic periodic paralysis. Cell. 1991. 67:1021–1027.

5. McClatchey AI, McKenna-Yasek D, Cros D, Worthen HG, Kuncl RW, DeSilva SM, et al. Novel mutations in families with unusual and variable disorders of the skeletal muscle sodium channel. Nat Genet. 1992. 2:148–152.

6. Orrell RW, Jurkat-Rott K, Lehmann-Horn F, Lane RJ. Familial cramp due to potassium-aggravated myotonia. J Neurol Neurosurg Psychiatry. 1998. 65:569–572.

7. Bulman DE, Scoggan KA, van Oene MD, Nicolle MW, Hahn AF, Tollar LL, et al. A novel sodium channel mutation in a family with hypokalemic periodic paralysis. Neurology. 1999. 53:1932–1936.

8. Tsujino A, Maertens C, Ohno K, Shen XM, Fukuda T, Harper CM, et al. Myasthenic syndrome caused by mutation of the SCN4A sodium channel. Proc Natl Acad Sci U S A. 2003. 100:7377–7382.
9. Cannon SC. Spectrum of sodium channel disturbances in the nondystrophic myotonias and periodic paralyses. Kidney Int. 2000. 57:772–779.

10. Moon IS, Kim HS, Shin JH, Park YE, Park KH, Shin YB, et al. Novel CLCN1 mutations and clinical features of Korean patients with myotonia congenita. J Korean Med Sci. 2009. 24:1038–1044.

11. Wu FF, Takahashi MP, Pegoraro E, Angelini C, Colleselli P, Cannon SC, et al. A new mutation in a family with cold-aggravated myotonia disrupts Na(+) channel inactivation. Neurology. 2001. 56:878–884.

12. Rosenfeld J, Sloan-Brown K, George AL Jr. A novel muscle sodium channel mutation causes painful congenital myotonia. Ann Neurol. 1997. 42:811–814.

13. Ptacek LJ, Gouw L, Kwieciński H, McManis P, Mendell JR, Barohn RJ, et al. Sodium channel mutations in paramyotonia congenita and hyperkalemic periodic paralysis. Ann Neurol. 1993. 33:300–307.

14. Plassart E, Eymard B, Maurs L, Hauw JJ, Lyon-Caen O, Fardeau M, et al. Paramyotonia congenita: genotype to phenotype correlations in two families and report of a new mutation in the sodium channel gene. J Neurol Sci. 1996. 142:126–133.

15. McClatchey AI, McKenna-Yasek D, Cros D, Worthen HG, Kuncl RW, DeSilva SM, et al. Novel mutations in families with unusual and variable disorders of the skeletal muscle sodium channel. Nat Genet. 1992. 2:148–152.

16. Lerche H, Heine R, Pika U, George AL Jr, Mitrovic N, Browatzki M, et al. Human sodium channel myotonia: slowed channel inactivation due to substitutions for a glycine within the III-IV linker. J Physiol. 1993. 470:13–22.

17. Mitrović N, George AL Jr, Lerche H, Wagner S, Fahlke C, Lehmann-Horn F. Different effects on gating of three myotonia-causing mutations in the inactivation gate of the human muscle sodium channel. J Physiol. 1995. 487:107–114.

18. Rüdel R, Lehmann-Horn F. Paramyotonia, potassium-aggravated myotonias and periodic paralyses. 37th ENMC International Workshop, Naarden, The Netherlands, 8-10 December 1995. Neuromuscul Disord. 1997. 7:127–132.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download