Abstract
Background and Purpose
Hyperglycemia after acute ischemic stroke (AIS) is associated with poor outcomes. However, there is no consensus as to the optimal method for glycemic control. We designed an insulin infusion protocol for aggressive glucose control and investigated its efficacy and safety.
Methods
We applied our protocol to patients within 48 hours after AIS or transient ischemic attack (TIA) with an initial capillary glucose level of between 100 and 399 mg/dL (5.6-22.2 mmol/L). An insulin solution comprising 40 or 50 U of human regular insulin in 500 mL of 5% dextrose was administered for 24 hours. Capillary glucose was measured every 2 hours and the infusion rate was adjusted according to a nomogram with a target range of 80-129 mg/dL (4.4-7.2 mmol/L). Changes in glucose and overall glucose levels during insulin infusion were analyzed according to the presence of diabetes or admission hyperglycemia (admission glucose >139 mg/dL or 7.7 mmol/L) by the generalized estimating equation method.
Results
The study cohort comprised 115 consecutive patients. Glucose was significantly lowered from 160±57 mg/dL (8.9±3.2 mmol/L) at admission to 93±28 mg/dL (5.2±1.6 mmol/L) during insulin infusion (p<0.05). Laboratory hypoglycemia (capillary glucose <80 mg/dL or 4.4 mmol/L) occurred in 91 (71%) patients, 11 (10%) of whom had symptomatic hypoglycemia. Although glucose levels were significantly lowered and maintained within the target range in all patients, overall glucose levels were significantly higher in patients with diabetes or hyperglycemia (p<0.05).
Poststroke hyperglycemia (PSH) refers to elevation of blood glucose during the acute period of stroke and can occur in patients either with or without diabetes.1 Depending on definitions and study methodologies, between 20% and 50% of acute stroke patients have been shown to have hyperglycemia at presentation.2,3 Although the importance of PSH is not well established and the role of glycemic intervention in PSH remains unclear, many studies have suggested an association between PSH and poor clinical outcomes.1,4-10 Intensive monitoring and control of physiologic parameters such as blood pressure and glucose is also cited as one of the benefits of acute stroke unit care.11 Various methods of acute glycemic intervention for PSH have been reported and there is presently no consensus as to the optimal method. In some studies, the impact of PSH on clinical outcomes was apparent in nondiabetic hyperglycemia,1,12 and one study found different therapeutic targets for diabetic and nondiabetic hyperglycemia.13 The method or intensity of glycemic control may also differ in patients with severe PSH. However, no study on acute glycemic intervention has addressed these issues.
As a part of critical pathway development for the management of patients with acute ischemic stroke (AIS) or transient ischemic attack (TIA), we developed a protocol for aggressive glucose control in AIS patients by insulin infusion (AGAIN). We examined the efficacy and safety of our protocol according to the presence of diabetes and the severity of admission hyperglycemia.
From January 2007, we developed a protocol using continuous insulin infusion for acute glycemic control in patients with AIS or TIA. For protocol development, we hypothesized that the glycemic response to insulin infusion would differ according to the presence of diabetes or the severity of hyperglycemia on admission. We therefore initially developed a single protocol for all patients, with the intention of amending it according to the results of a pilot study. We prospectively applied the single protocol to patients (aged 35-90 years) with AIS or TIA within 48 hours of symptom onset who had an admission capillary glucose level of 100-399 mg/dL (5.6-22.2 mmol/L). Patients with type 1 diabetes, diabetic ketoacidosis or nonketotic hyperosmolar state, heart failure, acute myocardial infarction, infection or high fever, severe anemia, renal failure, or other conditions that the duty neurologist judged inappropriate for the protocol were excluded. Patients with dementia, decreased consciousness, aphasia, severe dysarthria, and those who could not be admitted to the intensive care unit were also excluded.
All enrolled patients fasted and were treated with our insulin-infusion protocol for 24 hours in the intensive care unit. Insulin infusion after 24 hours was at the duty doctor's discretion and data obtained after 24 hours were not included in this analysis. During insulin infusion, all premorbid antidiabetic medications were temporarily discontinued. The initial protocol utilized an infusion of 500 mL of 5% dextrose with 50 U of human regular insulin (Humulin R, Lilly Korea, Seoul, Korea) and 20 mmol of potassium chloride (KCl) starting at the rate of 20 mL/hour (2.0 U/hour human regular insulin). We subsequently modified our protocol to reduce the amount of insulin from 50 to 40 U (Table 1) due to concerns about hypoglycemia. Although the decision to apply the protocol was based on glucose levels on admission, baseline capillary glucose was also measured just before the start of insulin infusion.
Capillary glucose was measured during insulin infusion every 2 hours. The infusion rate was changed according to the dose adjustment nomogram of the protocol so as to maintain the capillary glucose at 80-129 mg/dL (4.4-7.2 mmol/L). We defined laboratory hypoglycemia as a capillary glucose level of less than 80 mg/dL (4.4 mmol/L), and defined symptomatic hypoglycemia as the appearance of any of the symptoms of hypoglycemia (decreased consciousness, generalized weakness, dizziness, and sweating). Vital signs and clinical status were monitored every hour and serum electrolytes including sodium and potassium were checked every 12 hours.
All clinical data including National Institutes of Health Stroke Scale (NIHSS) score during admission and modified Rankin Scale (mRS) score at 3 months were prospectively registered on the Eulji Stroke Registry, which is incorporated with a Web-based stroke registry of Clinical Research Center for Stroke.14 Data on glucose measurements and hypoglycemia during insulin infusion were recorded prospectively in a separate AGAIN database using Microsoft Office Access 2003 software.
Based on the initial hypothesis, all patients were assigned to either the diabetic or nondiabetic group, and the hyperglycemic (admission capillary glucose >139 mg/dL or 7.7 mmol/L) or normoglycemic (admission capillary glucose from 100-139 mg/dL or 5.6-7.7 mmol/L) group. Efficacy was evaluated for initial glycemic response at 2 hours after infusion, and the changes in glucose and overall glucose levels during insulin infusion. Safety was evaluated for the incidence of laboratory and symptomatic hypoglycemia.
Student's t-test for continuous variables and χ2 test for categorical variables were used for statistical analysis. Changes in glucose and overall glucose levels during insulin infusion was compared for capillary glucose measured every 2 hours using the generalized estimating equation method with baseline capillary glucose as a covariate. SAS version 9.1 was used for statistical analysis. Except where stated otherwise, the data are presented as mean±SD values.
Our protocol was developed to improve clinical practice rather than for academic purposes, and so we neither applied for the approval of the institutional review board of our hospital nor obtained written informed consent to participate from the patients.
From July 2007 to February 2009, 115 consecutive patients were treated with our insulin-infusion protocol. Baseline characteristics are listed in Table 2. The mean age of the study cohort was 67 years, and 70 of them (60.9%) were male. One hundred (87%) patients had stroke, 47 (41%) had diabetes, and 57 (50%) had hyperglycemia on admission. Compared to admission glucose, baseline glucose increased by 15±12 mg/dL (8.3±0.7 mmol/L) in 17 (15%) patients, decreased by 41±42 mg/dL (2.3±2.3 mmol/L) in 87 (76%) patients, and remained the same in 11 (6%) patients. Baseline glucose was below 100 mg/dL (5.6 mmol/L) in 20 (17%) patients, of whom 5 had diabetes and 7 had hyperglycemia on admission.
Thirty-eight and 77 patients were treated with infusates including 50 and 40 U of insulin, respectively. The glucose level during the 24 hours of insulin infusion was 93±28 mg/dL (5.2±1.6 mmol/L), which was significantly lower than that measured on admission (p<0.05). Hypoglycemia occurred in 91 (79.1%) patients and symptomatic hypoglycemia occurred in 11 (9.6%) patients, 6 (5.2%) of whom experienced recurrent symptomatic hypoglycemia. Insulin infusion was discontinued in four (3.5%) patients due to recurrent symptomatic hypoglycemia in three and decreased consciousness by stroke progression with persistent hyperglycemia in one patient. Thirty (79%) of 38 patients treated with 50 U of insulin and 61 (79%) of 77 patients treated with 40 U of insulin suffered laboratory hypoglycemia. The initial glycemic response at 2 hours after insulin infusion was differed significantly depending upon the presence of diabetes or hyperglycemia on admission (Table 3, Fig. 1). More patients with diabetes or hyperglycemia had capillary glucose levels above the target range, while more patients without diabetes or hyperglycemia had capillary glucose levels within or below the target range (p<0.05). The overall incidence of laboratory hypoglycemia was significantly higher in patients without hyperglycemia on admission (Table 3).
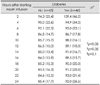
One hundred and eleven patients completed the protocol and were analyzed for changes in glucose and overall glucose levels during the 24 hours of insulin infusion, according to the presence of diabetes and hyperglycemia on admission (Table 4 and 5, Figs. 2 and 3). Glucose levels during insulin infusion changed significantly all groups (p<0.05 for a time effect). The interaction between change over time and groups was not significant (p>0.1 for an interaction effect between time and group), which means that changes in glucose levels over time were similar between comparison groups. Although mean glucose levels during insulin infusion were within the target range in all groups, the levels were significantly higher in patients with diabetes or hyperglycemia on admission (p<0.05 for a group effect).
On clinical assessment, early neurological deteriorationdefined as an increase in the NIHSS score of 2 or more at 72 hours after admission-occurred in 16 (13.9%) patients. The mRS score at 3 months was available in 97 (84%) patients, 68 (70.1%) of whom had a favorable outcome (mRS score ≥2). The occurrence of neurologic deterioration or favorable outcome at 3 months did not differ between patients with and without diabetes or with and without hyperglycemia on admission (p>0.1).
Despite the high prevalence of PSH, evidence regarding the effects of glycemic control is still lacking. Although current guidelines agree that PSH is associated with poor outcomes, there is no consensus on the frequency of monitoring, thresholds for intervention, or method of glycemic control.15,16 Several investigators have recently reported on the efficacy and safety of glycemic control in AIS patients using different methods.17-20 However, there is a wide variation in current clinical practice. We developed our own protocol and were able to achieve effective glycemic control in patients with AIS or TIA. Based on our initial hypothesis, we assessed the glycemic response to our protocol according to the presence of diabetes or the severity of hyperglycemia measured on admission.
There are several differences between our protocol and those described previously. For more stable glycemic control, all of our patients were ordered to fast during insulin infusion and we used 5% dextrose solution instead of normal saline. Unlike the method used in the United Kingdom Glucose Insulin in Stroke Trial,19 which was quite labor-intensive, insulin dose adjustment was achieved by simply changing the infusion rate according to the nomogram. As a result, overall glucose levels were well maintained within the target range in all patients. Although laboratory hypoglycemia was quite common, symptomatic hypoglycemia occurred in less than 10% of our patients. The high prevalence of laboratory hypoglycemia can be attributed to several features of our study. The definition of laboratory hypoglycemia (<80 mg/dL or 4.4 mmol/L) was more rigorous than that of other studies, in which it was defined as less than 54-72 mg/dL (3-4 mmol/L).17-19 In fact, despite the high incidence of laboratory hypoglycemia, the rate of symptomatic hypoglycemia in our study was comparable to those reported in other studies.17-19
The severity of hyperglycemia in our study patients is another consideration. We did not apply our protocol to patients who could not communicate properly due to altered consciousness, aphasia, or severe dysarthria, thus excluding patients with moderate-to-severe stroke. As a result, most patients had only mild-to-moderate hyperglycemia on admission. We also included TIA patients in whom glucose might decrease rapidly and significantly without insulin infusion as symptoms were resolving. A spontaneous decrease in glucose levels between admission and baseline measurements was also common, being observed in 76% of patients. Because the decision to apply the insulin-infusion protocol was based on the level of blood glucose at admission, insulin infusion was carried out even though the baseline glucose was less than 100 mg/dL (5.6 mmol/L), which occurred in 17% of study patients. Inclusion of patients who had admission glucose levels that were already within the target range (i.e., 100-130 mg/dL or 5.6-7.2 mmol/L) should also be considered. We included those patients because late hyperglycemia can occur more than 48 hours after stroke.5
Fasting during insulin infusion is another important factor. As mentioned above, all patients in our study fasted during insulin infusion because we thought that fasting for a short period after acute stroke is tolerable and can offer more stable glycemic control. Fasting is not mandatory in the general management of acute stroke if the patient does not suffer dysphagia. However, neurological deficit is unstable in the acute period and oral intake should be cautiously monitored because of the risk of aspiration. Although oral intake might have reduced the occurrence of hypoglycemia, it could also have caused fluctuations in blood glucose, thus rendering glycemic control more difficult and complicated.
In general, selecting the appropriate target range is important for optimizing the efficacy and safety of treatment. There is currently no consensus on these issues for glycemic control in acute stroke patients.4 In an animal experiment, glucose levels exhibited a U-shaped association with cortical necrosis and total infarction, with a nadir for cerebral necrosis in the 108-126 mg/dL (6-7 mmol/L) range.21 In humans, most studies addressing the association between glucose levels and poor outcome used predefined and arbitrary thresholds of 108-150 mg/dL (6-8.3 mmol/L).5-10 A meta-analysis revealed an association between admission glucose levels higher than 110-126 mg/dL (6.1-7.0 mmol/L) and increased risk of mortality in nondiabetic ischemic stroke patients. A recent study found that the optimal cutoff level for poor outcome at 3 months was 155 mg/dL (8.5 mmol/L).22 In glycemic intervention studies after AIS, the lower thresholds for inclusion ranged from 108 to 170 mg/dL (6.0-9.4 mmol/L) and target ranges were usually between 70 and 130 mg/dL (3.9 and 7.2 mmol/L).17-19 This variability was attributable to differences in patient populations (differences in stroke subtypes, diabetic population, and time window after stroke). In a large study involving 1,259 patients with AIS, hyperglycemia was associated with worse clinical outcomes only in nonlacunar stroke.23 Because hyperglycemia decreases over time after stroke,24 including patients visiting during the late time window may influence the threshold level for poor outcomes.
There is evidence that the benefit of glycemic control or the optimal range of glucose concentration differs between diabetic and nondiabetic hyperglycemia.1,13 To address this issue, different approaches using different regimens and comparative analysis according to the patient characteristics are needed. In our analyses we found that the treatment effect of our protocol did not differ with the presence of diabetes and hyperglycemia on admission. Capillary glucose levels during insulin infusion decreased significantly and were maintained within the target range. However, although the difference was small, overall glucose levels during insulin infusion were persistently higher in patients with diabetes or hyperglycemia on admission. Considering that most patients had mildto-moderate hyperglycemia, this finding suggests that patients with severe diabetic hyperglycemia exhibit a different glycemic response.
More intensive control may be needed for patients treated with thrombolysis. Several studies involving patients treated with thrombolytic therapy have demonstrated a profound effect of hyperglycemia on outcomes.25-27 As mentioned above, the time window after stroke should also be considered. Unlike other studies,17-20 we extended the treatment time window to 48 hours after symptom onset. It has been shown that PSH is prolonged despite current guidelines-based treatment, and two hyperglycemic phases were identified: an early hyperglycemia phase within the first 8 hours and a later phase 48-88 hours poststroke.5 The timing of glycemic control after stroke may not necessarily follow conventional windows for other acute therapies, and patients may be potential candidates for benefit at 12-24 hours after acute stroke.
In conclusion, our insulin-infusion protocol was feasible and could achieve successful glycemic control in AIS or TIA patients with mild-to-moderate hyperglycemia. However, laboratory hypoglycemia was common, and differed significantly between patients with and without diabetes or admission hyperglycemia during treatment. To improve the efficacy and safety of this protocol, further modification is needed and tailored intervention should be considered, especially for patients with severe diabetic hyperglycemia.
Figures and Tables
Fig. 1
The distribution of capillary glucose at 2 hours after insulin infusion with regard to target range (TR).

Fig. 2
Change of mean capillary glucose during insulin infusion according to the presence of diabetes.

Fig. 3
Change of mean capillary glucose during insulin infusion according to the presence of admission hyperglycemia.

Table 3
Capillary glucose at 2 hours after insulin infusion, and incidence of hyperglycemia

Data are number of patients (% of patients in each column).
*Results of capillary glucose measured at 2 hours after insulin infusion, †p<0.05 for comparisons between patients with and without diabetes or with and without hyperglycemia on admission, ‡Target range, 80-129 mg/dL (4.4-7.2 mmol/L), §p>0.1 for comparison between patients with and without diabetes, ¶p<0.05 for comparison between patients with and without hyperglycemia on admission, **p>0.1 for comparisons between patients with and without diabetes or with and without hyperglycemia on admission. TR: target range.
Table 4
Levels of capillary glucose [mg/dL, mean (SD)] measured every 2 hours during insulin infusion with comparison according to the presence of diabetes (n=111)
Acknowledgements
This study was supported by 2004 Eulji Research Grant (EIRG-05-020-11E30).
This study was sponsored by Sanofi-Aventis Korea and Boryung Co., Ltd
References
1. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001. 32:2426–2432.

2. Scott JF, Robinson GM, French JM, O'Connell JE, Alberti KG, Gray CS. Prevalence of admission hyperglycaemia across clinical subtypes of acute stroke. Lancet. 1999. 353:376–377.

3. Toni D, Sacchetti ML, Argentino C, Gentile M, Cavalletti C, Frontoni M, et al. Does hyperglycaemia play a role on the outcome of acute ischaemic stroke patients? J Neurol. 1992. 239:382–386.
4. Quinn TJ, Lees KR. Hyperglycaemia in acute stroke--to treat or not to treat. Cerebrovasc Dis. 2009. 27:Suppl 1. 148–155.

5. Allport L, Baird T, Butcher K, Macgregor L, Prosser J, Colman P, et al. Frequency and temporal profile of poststroke hyperglycemia using continuous glucose monitoring. Diabetes Care. 2006. 29:1839–1844.

6. Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003. 34:2208–2214.

7. Kiers L, Davis SM, Larkins R, Hopper J, Tress B, Rossiter SC, et al. Stroke topography and outcome in relation to hyperglycaemia and diabetes. J Neurol Neurosurg Psychiatry. 1992. 55:263–270.

8. Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ. 1997. 314:1303–1306.

9. Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, et al. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology. 2002. 59:67–71.

10. Gentile NT, Seftchick MW, Huynh T, Kruus LK, Gaughan J. Decreased mortality by normalizing blood glucose after acute ischemic stroke. Acad Emerg Med. 2006. 13:174–180.

11. Bhalla A, Wolfe CD, Rudd AG. Management of acute physiological parameters after stroke. QJM. 2001. 94:167–172.

12. Stead LG, Gilmore RM, Bellolio MF, Mishra S, Bhagra A, Vaidyanathan L, et al. Hyperglycemia as an independent predictor of worse outcome in non-diabetic patients presenting with acute ischemic stroke. Neurocrit Care. 2009. 10:181–186.

13. Farrokhnia N, Björk E, Lindbäck J, Terent A. Blood glucose in acute stroke, different therapeutic targets for diabetic and non-diabetic patients? Acta Neurol Scand. 2005. 112:81–87.

14. Clinical Research Center for Stroke. Available at:
http://www.strokecrc.or.kr/.
15. European Stroke Organisation (ESO) Executive Committee. ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008. 25:457–507.
16. Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007. 38:1655–1711.

17. Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, et al. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke. 2008. 39:384–389.
18. Bruno A, Saha C, Williams LS, Shankar R. IV insulin during acute cerebral infarction in diabetic patients. Neurology. 2004. 62:1441–1442.

19. Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol. 2007. 6:397–406.

20. Walters MR, Weir CJ, Lees KR. A randomised, controlled pilot study to investigate the potential benefit of intervention with insulin in hyperglycaemic acute ischaemic stroke patients. Cerebrovasc Dis. 2006. 22:116–122.

21. Zhu CZ, Auer RN. Optimal blood glucose levels while using insulin to minimize the size of infarction in focal cerebral ischemia. J Neurosurg. 2004. 101:664–668.

22. Fuentes B, Castillo J, San José B, Leira R, Serena J, Vivancos J, et al. The prognostic value of capillary glucose levels in acute stroke: the GLycemia in Acute Stroke (GLIAS) study. Stroke. 2009. 40:562–568.

23. Bruno A, Biller J, Adams HP Jr, Clarke WR, Woolson RF, Williams LS, et al. Acute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurology. 1999. 52:280–284.

24. Gray CS, Hildreth AJ, Alberti GK, O'Connell JE. GIST Collaboration. Poststroke hyperglycemia: natural history and immediate management. Stroke. 2004. 35:122–126.
25. Alvarez-Sabin J, Molina CA, Montaner J, Arenillas JF, Huertas R, Ribo M, et al. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator--treated patients. Stroke. 2003. 34:1235–1241.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download