Abstract
Background
Multiple sclerosis (MS) is a demyelinating disease of the central nervous system. Secondary amyloidosis can occur as a complication of chronic systemic inflammatory and infectious diseases. Until now there has been no report of secondary amyloidosis associated with MS. We report herein a case of renal biopsy-proven secondary amyloidosis in a patient with MS.
Case Report
A 41-year-old woman with MS was hospitalized due to aggravated quadriparesis and edema in both lower extremities. Laboratory findings showed nephrotic-range proteinuria and hypoalbuminemia. A percutaneous renal biopsy procedure was performed, the results of which revealed secondary amyloid-A-type amyloidosis associated with MS.
Multiple sclerosis (MS) is a demyelinating autoimmune disease of the central nervous system. Its pathological triad comprises central nervous system inflammation, demyelination, and gliosis.1 Secondary amyloidosis, which develops secondarily to chronic inflammatory conditions such as rheumatoid arthritis, is now called amyloid-A (AA) amyloidosis because a major factor in the protein deposition process involves a cleaved product of the acute-phase protein, serum amyloid A (SAA).2 Cerebrovascular amyloid deposits in the region of demyelinated plaques without systemic amyloidosis have been reported rarely in cases of MS;3 however, there is no report in the literature of MS related to AA amyloidosis. This article presents a case of MS with secondary AA amyloidosis, presenting with nephrotic syndrome. This is the first report of secondary amyloidosis associated with MS.
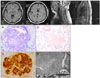
A 41-year-old Korean woman was hospitalized due to aggravated quadriparesis. She had been diagnosed with MS at an age of 33 years. At 26 years of age, the patient experienced her first episode of quadriparesis with sensory changes in both lower extremities. At 33 years, she noticed decreased visual acuity for several days. At 40 years, she was admitted to the hospital due to quadriparesis, dysarthria, and confusion, and her brain MRI showed brain, medullary, and spinal cord lesions (Fig. 1A) ; steroid pulse therapy was conducted. The patient's neurological symptoms improved, and she was discharged.
One year later, she was hospitalized again due to aggravated quadriparesis; she also complained of edema in both lower extremities. Steroid pulse therapy was performed again and the motor weakness in her upper extremities improved, but she continued to complain of dyspnea, orthopnea, and peripheral edema. Her chest X-ray showed cardiomegaly with pulmonary edema, but her echocardiogram showed normal findings. Laboratory studies showed the following parameters: white blood cell count 6,500/mm3, red blood cell count 2.93×106/mm3, hemoglobin 9.6 g/dL, hematocrit 28.1%, platelets 248,000/mm3, serum sodium 144 mEq/L, serum potassium 4.7 mEq/L, serum chloride 109 mEq/L, serum creatinine 1.2 mg/dL, serum blood urea nitrogen 78 mg/dL, serum albumin 2.5 g/dL, serum total protein 5.0 g/dL, serum cholesterol 218 mg/dL, and serum SAA 44.4 µg/mL (reference level <8 µg/mL). In addition, anti-nuclear antibody, anti-neutrophil cytoplasmic antibody, rheumatoid factor, and cryoglobulin were not detected in this patient's serum, which was also negative for hepatitis B surface antigen and anti-hepatitis-C antibody.
The 24-hour urinalysis revealed a protein excretion of 4,831 mg/day. A percutaneous renal biopsy procedure confirmed the presence of AA-type amyloidosis (Fig. 1B-E). The patient had no family history of amyloidosis. We performed immunofixation electrophoresis on her serum and urine to exclude a plasma-cell dyscrasia. M-proteins were not detected and hypoalbuminemia was observed using serum immunofixation electrophoresis. Urine immunofixation electrophoresis revealed albuminuria and increased globulin without an M-spike. AA amyloidosis was confirmed in view of the positive immunohistochemical staining for amyloid A and negative staining for kappa and lambda; however, with the exception of a past history of non-febrile asymptomatic bacteriuria and a short-lasting minor decubitus sore on the coccyx, there was no evidence of other systemic infection or inflammatory disease. Consequently, this case was diagnosed as secondary AA-type amyloidosis associated with MS.
We present herein the clinicopathological findings of a patient with MS who developed AA amyloidosis approximately 15 years after the onset of MS. Amyloidosis is caused by the extracellular deposition of pathologic, insoluble, fibrillar proteins in organs and tissues. Precursor proteins are known to change into fibrils through multiple mechanisms that differ among the various types of amyloid. Secondary amyloidosis is caused by the deposition of amyloid originating from SAA, which is an acute-phase protein produced in response to inflammation4 and occurs most commonly among patients with chronic inflammatory diseases such as rheumatoid arthritis, juvenile rheumatoid arthritis, and inflammatory bowel disease.5 Familial Mediterranean fever (FMF) is also an inflammatory disease characterized by episodic fever and serositis. Livneh et al.6 reported the development of AA amyloidosis in up to 90% of untreated FMF patients. A high prevalence of FMF was recently reported in one MS cohort in Turkey.7 These data suggest that MS is related to secondary amyloidosis, although until now there have been no reports of secondary amyloidosis in patients with MS.
One previous study found that SAA levels were increased in the peripheral blood of patients with relapsing-remitting type MS. SAA plays an important role in the conversion of innate immunity into the acquired immune response present during periods of acute and chronic inflammation.8 Therefore, increases in levels of SAA in MS patients may be considered evidence of the role of inflammation in MS, and may be a precursor of amyloid fibrils. However, there are no reports of increased levels of SAA in asymptomatic bacteriuria or decubitus sores.
Amyloid may be deposited either locally or systemically. The precursor proteins differ from each other in their primary structure and function. The clinical presentations and symptoms dependupon the distribution pattern and the amount of amyloid deposited. Renal involvement, as in this case, can cause nephrotic syndrome. The main treatment protocol for AA amyloidosis is management of the underlying inflammatory disease process, which usually focuses on the surgical debridement of inflammatory tissue, antibiotic treatment of infectious processes, anti-inflammatory medications (colchicine and anti-tumor necrosis factor blockade), and immunosupp ressive (cyclophosphamide) agents.9 However, these treatments, have not yet been established in randomized controlled studies. In our case, disease-modifying agents for MS (e.g., interferon-β, glatiramer acetate) could be considered for the treatment of MS-associated secondary amyloidosis. However, the effect of these agents has not yet been established.
There have been two previous reports of localized amyloid deposits in MS;5 however, there have been no reported cases of associated systemic amyloidosis. This is the first published case of secondary amyloidosis presenting as nephrotic syndrome associated with MS.
The findings of this case suggest strongly that MS is a chronic inflammatory disease and that secondary amyloidosis can develop in MS.
Figures and Tables
Fig. 1
MRI and microscopic findings. A: Fluid-attenuated inversion recovery sequence on MRI showing three plaques in the brain, and T2-weighted MRI showing severe atrophy of the cervical and thoracic cord. B: On light microscopy, the glomerulus exhibits extensive effacement of the glomerular architecture by large, irregularly shaped, amorphous, weakly periodic acid-Schiff (PAS)-positive, amyloid deposits (PAS ×400). C: Strongly Congoredpositive materials can be seen in the glomeruli and arterioles (Congoed, ×400). D: Amyloid deposits are positive on immunohistochemical staining for AA. E: Note the large nodular mesangial deposition of nonbranching, thin fibrils, ranging from 8.33 to 13.1 nm in thickness (electron micrograph, ×25,000).
References
2. Uda H, Yokota A, Kobayashi K, Miyake T, Fushimi H, Maeda A, et al. Two distinct clinical courses of renal involvement in rheumatoid patients with AA amyloidosis. J Rheumatol. 2006. 33:1482–1487.
3. Schroöder R, Nennesmo I, Linke RP. Amyloid in a multiple sclerosis lesion in clearly of Alambda type. Acta Neuropathol. 2000. 100:709–711.
5. Lachmann HJ, Goodman HJ, Gilbertson JA, Gallimore JR, Sabin CA, Gillmore JD, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007. 356:2361–2371.

6. Livneh A, Zemer D, Langevitz P, Laor A, Sohar E, Pras M. Colchicine treatment of AA amyloidosis of familial Mediterranean fever. An analysis of factor affecting outcome. Arthritis Rheum. 1994. 37:1804–1811.
7. Tuglular S, Yalcinkaya F, Paydas S, Oner A, Utas C, Bozfakioglu S, et al. A retrospective analysis for aetiology and clinical findings of 287 secondary amyloidosis cases in Turkey. Nephrol Dial Transplant. 2002. 17:2003–2005.

8. Ristori G, Laurenti F, Stacchini P, Gasperini C, Buttinelli C, Pozzilli C, et al. Serum amyloid A protein is elevated in relapsing-remitting multiple sclerosis. J Neuroimmunol. 1998. 88:9–12.

9. Schwimmer JA, Joseph RE, Appel GB. Wilcox CS, Brady HR, editors. Amyloid, fibrillary, and the glomerular deposition disease in therapy. Nephrology and Hypertension. 2003. Philadelphia: Saunders;253–261.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download