Abstract
Background and Purpose
Low levels of soluble receptor for advanced glycation end products (sRAGE) are associated with three conventional vascular risk factors (3Fs: diabetes, hypertension, and hypercholesterolemia), nondiabetic coronary artery disease, and Alzheimer's disease. However, the association between sRAGE and acute ischemic stroke (AS), especially AS without a source of cardioembolism, has not yet been established.
Methods
Patients with AS without a source of cardioembolism (n=259) and age-matched controls (n=300) were grouped according to the presence of 3Fs: AS patients with and without 3Fs (3Fs+ AS and 3Fs- AS, respectively) and controls with and without 3Fs (3Fs+ control and 3Fs- control, respectively). Levels of sRAGE were analyzed among the four groups.
Results
sRAGE was significantly higher in the controls than in the AS patients (855 pg/mL vs. 690 pg/mL, p<0.01). sRAGE was significantly higher in 3Fs- controls (996 pg/mL, p<0.05) than in 3Fs+ controls (721 pg/mL), and in AS group regardless of the 3Fs (629 pg/mL in 3Fs- and 705 pg/mL in 3Fs+). The lowest tertile of sRAGE was associated with an increased risk of AS in the 3Fs- group [adjusted odds ratio (OR) 4.0, 95% confidence interval (CI) 1.6-10.3, p<0.01] but not in the 3Fs+ group. The level of sRAGE was also correlated with neurological severity in the 3Fs- AS group (r=-0.32, p<0.05) but not in the 3Fs+ AS group.
The receptor for advanced glycation end products (RAGE) is a multiligand cell-surface protein that is expressed various cell types including endothelial cells and neurons.1,2 The binding of a ligand to RAGE stimulates the expression of RAGE itself, induces oxidative stress, and effectively modulates several steps of atherogenesis in diabetes.3-5 Recent studies have also demonstrated an increased expression of the cell-surface RAGE in dying neurons after hypoxic-ischemic insults and human cerebral ischemia, and suggested that the RAGE-ligand interaction causes neuronal cytotoxicity.6,7
RAGE also has a circulating truncated variant isoform, soluble RAGE (sRAGE), which corresponds to its extracellular domain only.4,5 Exogenously administered sRAGE has been successfully used to antagonize advanced glycation end products (AGE)-RAGE-mediated vascular damage.8 Accordingly, sRAGE may compete with cell-surface RAGE for the ligand, thus functioning as a decoy and possibly exerting a cytoprotective effect.4,5,8
Diabetes mellitus and hypertension are conventional risk factors for stroke, and hypercholesterolemia is related to vascular atherothrombosis. Decreased levels of sRAGE are found in diabetes mellitus, hypertension, hypercholesterolemia, nondiabetic coronary artery disease, and Alzheimer's disease.9-13 However, it is not yet known whether patients with acute is chemic stroke (AS), and especially AS without a source of cardioembolism, have lower levels of sRAGE compared to healthy subjects, or how plasma sRAGE levels differ according to the presence of any of the three conventional vascular risk factors (3Fs: diabetes mellitus, hypertension, and hypercholesterolemia).
To address these issues, we measured the levels of sRAGE in AS without source of cardioembolism and compared them with levels in age matched controls. In addition, we analyzed sRAGE in relation to any of the 3Fs. We next explored the relation between sRAGE and atherosclerosis [measured as carotid intimal medial thickness (IMT)] and stroke severity.
We prospectively evaluated 1,582 consecutive patients with a suspicion of AS who were admitted to the stroke unit of the department of neurology in our hospital between January 2006 and August 2008. For these, clinical information including demographic data, medical history, vascular risk factors, routine blood tests, neurologic examinations, chest radiography, and electrocardiography were obtained, and magnetic resonance imaging (MRI) and/or computer tomography (CT) of the brain were performed to screen for AS. All of the patients with AS (n=1,427) who had relevant lesions on MRI and/or CT underwent magnetic resonance angiography or distal subtraction angiography; when clinically indicated, some patients also underwent transesophageal echocardiography and/or Holter monitoring. Patients were then further classified according to the criteria of the Trial of Org 10172 in Acute Stroke Treatment (TOAST),14 which defines five etiologic subtypes of stroke: 1) large artery atherosclerosis or atherothrombosis (LAA), 2) small-vessel occlusion or lacunar (SVO), 3) cardioembolic (CE), 4) undetermined etiology or cryptogenic, and 5) other determined etiology.
Of the 1,427 AS patients, 329 fulfilled the following inclusion criteria: 1) first two subtypes (LAA and SVO) of first-ever AS, 2) 40-79 years of age at the onset of stroke, and 3) admission within the first 48 h after symptom onset. Seventy patients were excluded as a result of the following exclusion criteria: 1) two or more causes of stroke (n=7), 2) presence of transient ischemic attack, intracerebral hemorrhage, postseizure neurological deficit, brain abscess, or tumor (n=9), 3) previous history of cerebrovascular disease, presence or history of thromboembolic events, peripheral vascular disease, or cardiovascular disease other than hypertension (n=18), 4) specified comorbidities [metastatic malignancy, renal disorder (serum creatinine >2.0 mg/dL), autoimmune disorder, or hematologic disorder] (n=15), 5) current steroid treatment or taking lipid-lowering medication during the previous 3 months (n=11), or 6) refusal to participate in the study (n=10). A final cohort of 259 cases of first-ever AS without a source of cardioembolism was enrolled in the study, of which 190 (73%) presented with SVO and 69 (27%) presented with LAA. For the control group, 394 age-matched volunteers were recruited from a population that attended a health screening in the health-promotion center of our hospital. These participants were required to have no neurological deficits or neurologic symptoms during the previous 3 months. The same exclusion criteria as for the AS group were applied to the control group, which results in 300 age-matched controls being enrolled in the study.
For each patient with AS without a source of cardioembolism, the severity of the neurological deficit was rated at the time of admission according to the National Institutes of Health Stroke Scale (NIHSS) score. Of the 259 patients with AS, 204 (79%) had mild (score <5) neurological deficits and 55 (21%) had moderate (score 5-10) to severe (score >10) neurological deficits.
Diabetes mellitus was considered present if the participant was normally medicated with insulin or an oral agent, or had a fasting glucose level of ≥126 mg/dL. Hypertension was defined in patients with a systolic blood pressure of ≥140 mmHg, a diastolic pressure of ≥90 mmHg (as evidenced by at least two consecutive blood pressure measurements after 20 min rest), currently taking antihypertensive medication, or a combination of the three. Hypercholesterolemia was defined by a total cholesterol level of ≥200 mg/dL.
Blood samples were collected into both plain and ethylenediaminetetraacetic acid (EDTA)-containing tubes after a 12-h overnight fast within the first 48 h poststroke, as close as possible to the time of admission. Blood samples were also taken from the control subjects after a 12-h overnight fast. All of the samples were centrifuged at 1,500×g for 15 min and immediately separated; the serum fraction was used for a lipid profile test. One plasma aliquot was used for a glucose test and the other was stored at -76℃ and used for sRAGE testing.
Lipid and glucose levels were measured by an automated chemical analyzer (Roche Diagnostics, Mannheim, Germany) using the enzymatic colorimetric and hexokinase method. Plasma total sRAGE was determined using a commercially available enzyme-linked immunoassay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol. Measurements were performed in duplicate and the results were averaged. The intra- and interassay coefficients of variation were 7% and 10%, respectively.
To assess atherosclerosis in AS, ultrasonographic scanning of the carotid arteries was performed using an echotomographic system (Aloka, Tokyo, Japan) with an electrical liner transducer (mid frequency 8.0 MHz) in clinically available AS (n=156). Scanning of the extracranial common carotid arteries, the carotid bulbs, and the internal carotid arteries in the neck was performed bilaterally. The site of greatest thickness including a plaque lesion was sought along the arterial walls. Three determinations of IMT were conducted at the site of the greatest thickness and two adjacent points (located 1 cm up stream and 1 cm downstream of the thickest point). These three determinations were averaged to form the mean IMT. The greatest value among the six mean IMTs (three from the left side and three from the right) was used as the representative value for each patient.
The study protocol conformed to the Institutional Guidelines for Human Research, and all participants provided written informed consent to participate before entry into the study.
The statistical package for SPSS 11.0 (SPSS, Chicago, IL, USA) was used for statistical analysis, with the level of statistical significance set at p<0.05. Plasma levels of sRAGE were compared after logarithmic transformation because the concentrations were not normally distributed. The mean sRAGE levels were compared using Student's t-test (control vs. AS) and analysis of variance (ANOVA), followed by a Scheffé post-hoc test (among the four subgroups defined by the presence of AS and any of the 3Fs). Categorical variables are presented as frequency counts, and intergroup comparisons were performed using χ2 tests.
All of the subjects were categorized into tertiles based on their plasma sRAGE level, and the classification was used to determine the odds ratio (OR) for AS by multiple logistic regression analysis with adjustment for age (in years), sex, 3Fs, current smoking, hyper-low-density lipoprotein cholesterolemia, hypo-high-density lipoprotein cholesterolemia, and hypertriglyceridemia.
The demographic characteristics of the subjects are presented in Table 1. The age and sex distributions did not differ significantly between the control and AS groups (p>0.05). The level of sRAGE was significantly higher in the control group than in the AS group (855 pg/mL vs. 690 pg/mL, respectively, p<0.01)(Fig. 1A). To determine the relationship between plasma sRAGE level and 3Fs, those are previously reported as having a inverse association with sRAGE, the control and AS groups were subdivided according to the presence of any of the 3Fs. sRAGE was significantly higher in 3Fs- controls than in 3Fs+ controls and in AS regardless of the 3Fs (Fig. 1B).
The question of whether plasma sRAGE can be used as a biomarker for the risk of AS was addressed by categorizing all of the subjects into tertiles of plasma sRAGE level (lowest tertile <427 pg/mL, middle tertile 427-780 pg/mL, and highest tertile ≥780 pg/mL). AS was more prevalent in the lowest tertile group, as shown in Fig. 2.
The adjusted OR was determined to evaluate sRAGE as an independent biomarker for the risk of AS. The OR of AS was slightly higher in the lowest and middle tertiles of sRAGE than in the highest tertile in the total group (Fig. 3A). To further clarify the association between sRAGE and AS, all of the participants were subgrouped according to the presence of any of the 3Fs. The association between low plasma sRA GE level and AS was prominent in the 3Fs- group (adjusted OR 4.0, 95% confidence interval 1.6-10.3, p<0.01)(Fig. 3B), and was uncertain in the 3Fs+ group (Fig. 3C).
The relationship between plasma sRAGE level and atherosclerosis or neurological severity in AS was assessed by calculating and comparing the correlation coefficients between sRAGE and carotid IMT, and sRAGE and NIHSS score. Carotid IMT was not significantly correlated with sRAGE in any of the AS groups (i.e., total AS, 3Fs+ AS, 3Fs- AS)(Fig. 4A). In addition, the NIHSS score was not significantly correlated with sRAGE in either the total or 3Fs+ AS groups; however, it was significantly correlated with sRAGE in the 3Fs- AS group (r=-0.32, p<0.05)(Fig. 4B).
This is the first clinical study to demonstrate that the plasma level of sRAGE is lower in AS patients than in subjects without stroke. We also found that a decreased plasma sRAGE is an independent biomarker for the risk of AS in 3Fs- subjects. These findings are of interest because they suggest that plasma sRAGE level can be used as an identifiable risk factor for AS in patients without proven vascular risk factors.
Two opposing hypotheses exist regarding the relationship between plasma sRAGE levels and vascular risk factors or disease: one state that they are negatively correlated and the other that they are positively correlated. Although the mechanism is not yet clear, the former, negative-correlation hypothesis suggests that cardiovascular risk factors or disease increase the clearance of AGE ligand/sRAGE complexes or inhibit the production of sRAGE.4,5,9 On the other hand, the positive-correlation hypothesis suggests that the disease process leads to an excess release of sRAGE from activated endothelial cells; thus, sRAGE may reflect tissue RAGE expression.15-17
In our comparison of AS patients and age-matched controls, we confirmed the occurrence of decreased plasma levels of sRAGE in 3Fs+ subjects in the control group, a finding that is consistent with previous studies.4,5,9 Also, our study demonstrated a decreased level of sRAGE in AS. More importantly, multiple regression analysis revealed that the adjusted OR for AS was higher for the lowest tertile of sRAGE than for the highest tertile, especially in the 3Fs- group. These findings indicate that decreased plasma levels of sRAGE are associated with conventional vascular risk factors, and that they are independently associated with AS.
Circulating sRAGE is generated via the cleavage of cell-surface RAGE or novel splice variants of RAGE.4,5 The C-terminal truncated novel splice variant of RAGE is called endogenous secretory RAGE (esRAGE). It has been reported that esRAGE has a ligand-binding domain that is significantly correlated with IMT.18-21 However, a recent study did not find any significant correlation between total sRAGE and IMT, a finding that is consistent with our present results.21,22 These different findings regarding IMT, of total sRAGE and esRAGE, we may consider the effect of other variant forms of RAGE those comprise more proportion of total sRAGE than esRAGE or insufficiently analyzed extent of general atherosclerosis (i.e., the extent of extracranial carotid atherosclerosis that is closely related to carotid IMT was calculated, but the extent of intracranial atherosclerosis was not quantitatively analyzed in our study).
The correlation between sRAGE and NIHSS score was significant in 3Fs- AS patients, which suggests that sRAGE contributes to the pathogenesis of AS in patients with no known vascular risk factors. There may be several reasons for the absence of no significant correlation between sRAGE and NIHSS score in 3Fs+. First, the medications used only by 3Fs+ subjects (e.g., diabetes and hypertension medications) may affect this relationship. sRAGE expression could be affected by medication dose rather than neurological deficit, especially in 3Fs+ AS patients. Second, the plasma sRAGE level may reflect the extent of vascular risk factors more potently than the severity of neurological deficit in the 3Fs+ group. For example, a mild neurological deficit with severe hypertension and severe diabetes may affect the circulatory levels of sRAGE more potently than a severe neurological deficit with mild diabetes. Finally, a masking effect caused by excess release of sRAGE from activated brain endothelial cells or dying neuronal cells may be related to destruction of the blood-brain barrier.6,7,23 In the present study, the portion of patients with severe neurological deficits (NIHSS score >10) was higher in the 3Fs+ AS group than in the 3Fs- AS group, and some of these severely deteriorated patients had relatively high sRAGE levels (Fig. 4B). Thus, we postulate that within a certain range, decreased levels of sRAGE reflect the severity of AS; however, above that range, the excess sRAGE that is released from dead or injured cells can mask the negative correlation between sRAGE and the severity of AS.
This study was subject to some limitations. First, because of the cross-sectional design, the causal relationship between low sRAGE concentration and AS and the mechanism by which plasma sRAGE levels are decreased in AS could not be determined. In particular, we could not determine whether sRAGE affects the future morbidity and severity of stroke in 3Fs+ subjects who had decreased levels of sRAGE before their strokes. Second, we excluded subjects who were taking statins during the 3 months before admission. Nonetheless, because of the clinical state of subjects, those with diabetes and/or hypertension who took medication for these diseases were included. Unfortunately, we did not analyze the effects of medications on the level of sRAGE.
Because of these important caveats, prospective longitudinal and intervening studies are required to confirm the role of sRAGE as a biomarker for the risk of AS and to clarify its pathophysiological effects on AS.
Overall, our results demonstrate that decreased plasma levels of sRAGE are associated with AS due to cerebrovascular atherosclerosis or 3Fs in stroke-free subjects, and that there is a negative correlation between sRAGE and neurological severity in 3Fs- AS. These findings suggest that decreased plasma sRAGE contributes to the pathogenesis of AS and is a candidate biomarker for the risk of AS, particularly in 3F- subjects who have no identifiable conventional risk factors for AS. Further longitudinal and integrated studies are required to define the role of sRAGE in the pathogenesis and neurological severity of 3Fs+ AS.
Figures and Tables
 | Fig. 1Comparison of soluble receptor for advanced glycation end products (sRAGE) levels according to the presence of acute ischemic stroke (AS)(A) or any of the three sRAGE-related vascular risk factors (3Fs)(B): diabetes, hypertension, and hypercholesterolemia. Data are mean and 95% confidence interval values. *t-test, †ANOVA test followed by a Scheffe post-hoc test. ns: not significant. |
 | Fig. 2Numbers of subjects across tertiles of sRAGE levels in controls (white bars) and AS patients (gray bars). sRAGE: soluble receptor for advanced glycation end products, AS: acute ischemic stroke. |
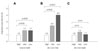 | Fig. 3Association between tertiles of sRAGE and risk of AS according to the presence of any of the 3Fs (A: 3Fs positive and negative group. B: 3Fs negative group. C: 3Fs positive group). The odds ratio was adjusted for age (in years), sex, 3Fs, current smoking, hyper-low-density lipoprotein cholesterolemia, hypo-high-density lipoprotein cholesterolemia, and hypertriglyceridemia. sRAGE: soluble receptor for advanced glycation end products, AS: acute ischemic stroke. |
 | Fig. 4Correlations between plasma sRAGE and carotid intimal-medial thickness (A) or National Institutes of Health Stroke Scale (NIHSS) score (B) according to the presence of any of the 3Fs in AS. sRAGE: soluble receptor for advanced glycation end products, AS: acute ischemic stroke. |
References
1. Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993. 143:1699–1712.
2. Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and coexpression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995. 270:25752–25761.

3. Stern D, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv Drug Deliv Rev. 2002. 54:1615–1625.

4. Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis. 2008. 196:9–21.

5. Koyama H, Yamamoto H, Nishizawa Y. RAGE and soluble RAGE: potential therapeutic targets for cardiovascular diseases. Mol Med. 2007. 13:625–635.

6. Ma L, Carter RJ, Morton AJ, Nicholson LF. RAGE is expressed in pyramidal cells of the hippocampus following moderate hypoxic-ischemic brain injury in rats. Brain Res. 2003. 966:167–174.

7. Zhai DX, Kong QF, Xu WS, Bai SS, Peng HS, Zhao K, et al. RAGE expression is up-regulated in human cerebral ischemia and pMCAO rats. Neurosci Lett. 2008. 445:117–121.

8. Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002. 106:2827–2835.

9. Basta G, Sironi AM, Lazzerini G, Del Turco S, Buzzigoli E, Casolaro A, et al. Circulating soluble receptor for advanced glycation end products is inversely associated with glycemic control and S100A12 protein. J Clin Endocrinol Metab. 2006. 91:4628–4634.

10. Geroldi D, Falcone C, Emanuele E, D'Angelo A, Calcagnino M, Buzzi MP, et al. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J Hypertens. 2005. 23:1725–1729.

11. Santilli F, Bucciarelli L, Noto D, Cefalù AB, Davì V, Ferrante E, et al. Decreased plasma soluble RAGE in patients with hypercholesterolemia: effects of statins. Free Radic Biol Med. 2007. 43:1255–1262.

12. Falcone C, Emanuele E, D'Angelo A, Buzzi MP, Belvito C, Cuccia M, et al. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005. 25:1032–1037.

13. Emanuele E, D'Angelo A, Tomaino C, Binetti G, Ghidoni R, Politi P, et al. Circulating levels of soluble receptor for advanced glycation end products in Alzheimer disease and vascular dementia. Arch Neurol. 2005. 62:1734–1736.

14. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993. 24:35–41.

15. Nakamura K, Yamagishi S, Adachi H, Kurita-Nakamura Y, Matsui T, Yoshida T, et al. Elevation of soluble form of receptor for advanced glycation end products (sRAGE) in diabetic subjects with coronary artery disease. Diabetes Metab Res Rev. 2007. 23:368–371.

16. Nakamura K, Yamagishi S, Nakamura Y, Takenaka K, Matsui T, Jinnouchi Y, et al. Telmisartan inhibits expression of a receptor for advanced glycation end products (RAGE) in angiotensin-II-exposed endothelial cells and decreases serum levels of soluble RAGE in patients with essential hypertension. Microvasc Res. 2005. 70:137–141.

17. Yamagishi S, Imaizumi T. Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) may reflect tissue RAGE expression in diabetes. Arterioscler Thromb Vasc Biol. 2007. 27:e32. author reply e33-e34.

18. Koyama H, Shoji T, Yokoyama H, Motoyama K, Mori K, Fukumoto S, et al. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005. 25:2587–2593.

19. Katakami N, Matsuhisa M, Kaneto H, Matsuoka TA, Sakamoto K, Nakatani Y, et al. Decreased endogenous secretory advanced glycation end product receptor in type 1 diabetic patients: its possible association with diabetic vascular complications. Diabetes Care. 2005. 28:2716–2721.

20. Katakami N, Matsuhisa M, Kaneto H, Yamasaki Y. Serum endogenous secretory RAGE levels are inversely associated with carotid IMT in type 2 diabetic patients. Atherosclerosis. 2007. 190:22–23.

21. Katakami N, Matsuhisa M, Kaneto H, Matsuoka TA, Sakamoto K, Yasuda T, et al. Endogenous secretory RAGE but not soluble RAGE is associated with carotid atherosclerosis in type 1 diabetes patients. Diab Vasc Dis Res. 2008. 5:190–197.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download