Abstract
The sirtuins (SIRTs) are protein-modifying enzymes that are distributed ubiquitously in all organisms. SIRT1 is a mammalian homologue of yeast nicotinamide-adenine-dinucleotide-dependent deacetylase silent information regulator 2 (known as Sir2), which is the best-characterized SIRT family member. It regulates longevity in several model organisms and is involved in several processes in mammalian cells including cell survival, differentiation, and metabolism. SIRT1 induction, either by SIRT-activating compounds such as resveratrol, or metabolic conditioning associated with caloric restriction, could have neuroprotective qualities and thus delay the neurodegenerative process, thereby promoting longevity. However, the precise mechanistic liaison between the activation of SIRT and extended healthy aging or delaying age-related diseases in humans has yet to be established.
Neurodegenerative disorders including Huntington's disease, Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), and Alzheimer's disease (AD) are characterized by irreversibility, a progressive clinical course, and idiopathic degeneration of specific selectively vulnerable neuronal populations. These debilitating neurodegenerative diseases are inherently associated with the accumulation of misfolded proteins that adversely affect neuronal connectivity and plasticity, and trigger cell-death-signaling pathways.1 However, the precise sequence of the events that underlie disease progression remains to be identified, and this largely explains the absence of methods and effective therapeutic interventions for this group of diseases. While the misfolded proteins typically exhibit loss of function, mislocalization, and tendency toward aggregation, most of these processes are strongly influenced by aging, which is the predominant and unifying risk factor for neurodegenerative diseases.
It is well established that low-calorie diets, known as "caloric restriction" (CR), extend lifespan in a wide variety of organisms including yeast, Caenorhabditis elegans, Drosophila species, and rodents, and it has been proposed that the sirtuins (SIRTs) might at least partly mediate this effect.2 Thus, activating molecular pathways that slow the process of aging may provide an outstanding strategy for treating and preventing these conditions. This is where SIRTs may come into play, which are nicotinamide adenine dinucleotide (NAD+)-dependent enzymes that have emerged as important regulators of diverse biological processes and are referred to as either SIRTs or silent information regulator 2 (Sir2)-like proteins. They constitute the class III histone deacetylases and are conserved from bacteria to humans.3 The founding member, yeast Sir2 (ySir2), is essential for maintaining silent chromatin through the deacetylation of histones. Since the discovery of the involvement of SIRT in apoptosis, cell survival, transcription, metabolism, and aging, these activities have been implicated as disease modifiers. This review highlights the role of SIRTs as potential therapeutic targets for developing treatments for neurodegenerative disorders. Although SIRT1 and SIRT2 play important roles in aging and neurodegeneration, very little is known about their role in the central nervous system (CNS). Therefore, following a brief description of the SIRTs in general, this review focuses on SIR1 and SIR2.
SIRTs, a family of NAD+-dependent deacetylases and/or adenosine diphosphate (ADP)-ribosyltransferases, are an evolutionarily conserved class of proteins that regulate various cellular functions such as genome maintenance, longevity, metabolism, and tolerance to oxidative stress.4-6 These enzymes were first identified in yeast as silent information regulators, hence the family name.7 SIRTs regulate cell functions by deacetylating both histone and nonhistone targets. Sir2 in Saccharomyces cerevisiae is the founding member of the SIRT gene family, and its deacetylase activity is required for chromatin silencing at the mating-type loci, telomeres, and the ribosomal DNA locus. Seven distinct Sir2 homologues have been identified in humans (SIRT1-SIRT7), each having distinct cellular targets and diverse cellular localizations. Robust protein deacetylase activity has been reported for SIRT1, SIRT2, SIRT3, and SIRT5, whereas SIRT4, SIRT6, and SIRT7 have no detectable enzymatic activity on a histone peptide substrate.8 The current consensus suggests that mammalian SIRTs comprise two nuclear (SIRT1, SIRT6), one cytoplasmic (SIRT2), three mitochondrial (SIRT3, SIRT4, and SIRT5), and one nucleolar (SIRT7) protein (Table 1).9
SIRT1, which is found predominantly in the nucleus, has the highest sequence homology to ySir2. An early insight into one mechanism whereby Sir2 could increase the replicative lifespan of yeast comes from the discovery that it acts at the nucleolus, inhibiting ribosomal DNA (rDNA) recombination as well as extrachromosomal rDNA circle formation.10 It is the best-investigated and most-well-understood member of the human family of SIRTs in terms of its endogenous function and activity, and is suggested to play an essential role in lifespan extension (on CR), the oxidative stress response [poly (ADP-ribose) polyMerase], and regulation of forkhead transcription factors (FOXOs) and p53. Other important substrates of SIRT1 include, Ku70, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), liver X receptor (LXR), and histones H1, H3, and H4, with histone deacetylation causing gene silencing.11 SIRT1 physically interacts with p53 in the nucleus, an interaction that is enhanced after the induction of DNA damage. Acetylation of p53 results in the activation of p53 target genes such as p21, resulting in cell-cycle arrest, apoptosis, or senescence. Conversely, deacetylation of p53 by SIRT1 decreases p53-mediated transcriptional activation.12 SIRT1 activity results in the suppression of apoptosis induced by DNA damage or oxidative stress (Fig. 1).
Since nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) exerts an antiapoptotic effect during tumor necrosis factor-α (TNF-α) activation, inhibition of NF-κB-mediated gene activation by SIRT1 sensitizes cells to apoptosis during TNF-α treatment. Ku70 is a subunit of the Ku protein complex, which is involved in the nonhomologous repair of DNA double-strand breaks. SIRT1 and Ku70 physically interact in vivo, and overexpression of SIRT1 decreases the acetylation level of Ku70, thereby promoting the antiapoptotic Bcl-2-associated X protein-Ku70 interaction.
Members of the FOXO family of transcription factors are involved in cellular processes that range from longevity, metabolism, and reproduction in C. elegans to the regulation of gene transcription downstream from insulin, cell-cycle arrest, apoptosis, and stress responses in mammalian cells, and acetylation, and function as transcriptional coactivators.13,14 SIRT1 interacts with FOXO proteins and deacetylates FOXO1, FOXO3, and FOXO4. It appears that SIRT1 shifts FOXO-mediated processes from induction of apoptosis to cell-cycle arrest and cellular survival.
In mouse embryos, SIRT1 was expressed at high levels in the heart, brain, spinal cord, and dorsal root ganglia.15 High SIRT1 levels in the embryonic brain suggest that it plays a role in neuronal and/or brain development. This notion is supported by some of the phenotypes associated with SIRT1-knockout mice, in which postnatal survival is infrequent, and which have developmental defects such as exencephaly and retinal anomaly.16 In the adult rat brain, SIRT1 can be found in the hippocampus, cerebellum, and cerebral cortex. The antioxidant vitamin E has been shown to reduce the oxidative damage and reduction of SIRT1 caused by a high-fat and high-sugar diet, while restoring SIRT1 levels.17
The findings of that study suggest that SIRT1 levels in the brain are affected by oxidative stress and energy homeostasis. There is also recent evidence that SIRT1 deacetylates autophagy genes and stimulates basal rates of autophagy,18 which has emerged as an important route for the removal of the toxic misfolded protein aggregates that accumulate in neurodegenerative diseases.
The human SIRT2 protein is a closer homologue to the yeast Hst2p than to ySir2. Both proteins are localized in the cytoplasmic compartment, but human SIRT2 is also localized along the microtubule network.19 SIRT2 has been reported to promote neuronal death. Pharmacological and genetic inhibition of SIRT2 protects neurons against α-synuclein toxicity both in vitro and in flies.20 In addition to deacetylating histone-H3 peptide acetylated on lysine-14, SIRT2 is capable of deacetylating an acetylated α-tubulin peptide, an ability that Hst2p clearly lacks. Hence, SIRT2 shows a preference for an α-tubulin peptide over a histone peptide, suggesting that SIRT2 has evolved to carry out the deacetylation of tubulin. As tubulin acetylation is implicated in the regulation of cell shape, intracellular transport, cell motility, and cell division, it will be of future interest to address the role of SIRT2 in tubulin deacetylation, as well as in the concept of CNS diseases. The SIRT2 gene is found at chromosome 19q13.2, a region that is frequently deleted in human gliomas. Furthermore, the ectopic expression of SIRT2 in a glioma cell line has been shown to decrease colony formation, suggesting a potential tumor-suppressor role for SIRT2. This could be explained by SIRT2 playing an important role in the control of mitotic exit in the cell cycle, where increased SIRT2 activity severely delays cell-cycle progression through mitosis.21 SIRT2 was very recently described as an oligodendroglial cytoplasmic protein localized to the outer and juxtanodal loops in the myelin sheath, and which decreases cell differentiation through α-tubulin deacetylation, suggesting a potential role in myelinogenesis.22
Many neurodegenerative disorders are characterized by conformational changes in proteins that result in misfolding, aggregation, and intra- or extraneuronal accumulation of amyloid fibrils. The variety and complexity of these diseases are related to the different pathological conformations that the proteins involved can assume. Most conformational diseases, such as AD, PD, and ALS, are caused by a combination of genetic and environmental factors, suggesting that spontaneous events can destabilize a misfolding-prone protein or impair the clearance mechanisms, leading to the accumulation of misfolded aggregates. While aging is a major risk factor because it may compromise both the cellular processing and clearance systems, environmental factors affect the probability of disease onset and progression.
The currently available therapeutic strategies are still not effective enough to slow or prevent these diseases; the development of new therapeutic approaches that specifically target the pathogenic proteins is therefore mandatory. Below we describe some of the representative neurodegenerative disorders that represent potential targets of SIRT-related mechanisms.
The histopathological hallmarks of AD are the presence of intraneuronal neurofibrillary tangles and the accumulation of extracellular amyloid plaques in the brains of affected individuals. A link between SIRT1 and AD is also becoming increasingly evident. NF-κB signaling in microglia is known to be critically involved in neuronal death induced by Aβ peptides.23
SIRT1 protects against Aβ-induced neurotoxicity by inhibiting NF-κB signaling in microglia. Overexpression of SIRT1 and resveratrol treatment has been shown to markedly reduce Aβ-stimulated NF-κB signaling and to exert a strong neuroprotective effect. This finding concurs with the known role of SIRT1 in modulating NF-κB activity.24 Shortterm CR was shown to substantially decrease the accumulation of Aβ plaques in two AD-prone amyloid precursor protein (APP)/presenilin transgenic mouse lines, and to decrease gliosis, as marked by astrocytic activation. The authors suggest that CR enhances the clearance of brain Aβ by reducing brain insulin as a competing substrate. The overexpression of SIRT1 or pharmacological activation of SIRT1 by NAD+ also promotes α-secretase activity and attenuates the generation of Aβ peptides in embryonic Tg2576 mouse neurons in vitro.
Moreover, in Tg2576 mice, CR resulted in a larger than twofold increase in the concentration of brain soluble APPα (a product of α-secretase cleavage of APP) and a statistically significant 30% increase in ADAM10 (A Disintegrin And Metallopeptidase 10, a putative α-secretase) levels in CR animals compared to controls.25 Other mechanisms could include lower cholesterol and higher glucocorticoid levels in CR mice.26 In a recent investigation using resveratrol, which is a well-known CR-mimicking agent, we found that Aβ-induced neurodegeneration was attenuated by the mechanisms involved in the 5' adenosine monophosphate-activated protein kinase (AMPK) pathways (unpublished data). It is thus possible that SIRT regulates one or more of the AMPK kinases.27 Another plausible explanation is the activation of SIRT1 by CR.
PD is characterized neuropathologically by the selective and progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta, which is accompanied by muscle rigidity, bradykinesia, resting tremor, and postural instability. There is a growing body of evidence that both genetic and environmental factors contribute to the acceleration of dopaminergic neurodegeneration in this neurological disorder. In particular, mitochondrial dysfunction has been considered one of the most important factors involved in the pathogenesis of PD. While misfolding, oligomerization, and aggregation of α-synuclein have been implicated in PD pathology, the precise mechanisms underlying the neurodegeneration remains to be determined.
Okawara et al.21 recently investigated whether resveratrol exhibits neuroprotective effects on dopaminergic neurons in organotypic midbrain slice cultures subjected to several different types of insult related to PD pathogenesis. They demonstrated that resveratrol, together with another SIRT-activating compound, quercetin, prevents the decrease of dopaminergic neurons induced by a dopaminergic neurotoxin 1-methyl-4-phenyl pyridinium. They suggested that resveratrol exerts neuroprotective effects in dopaminergic neurons via either antioxidative or SIRT-activating activity. Moreover, Outeiro et al.28 recently described the identification and characterization of SIRT2 inhibitors and demonstrated that pharmacological and genetic inhibition of SIRT2 rescues cell cultures from α-synuclein toxicity. However, it is still unclear whether it is the antioxidant or SIRT-activating activity (or both) that underlies this neuroprotective effect of resveratrol.
ALS is an adult-onset neurodegenerative disease characterized by the selective vulnerability of motor neurons in the spinal cord, brainstem, and motor cortex, causing progressive muscle weakness, atrophy, paralysis, and bulbar dysfunction, and leads to death within 3-5 years of disease onset in most cases. The sporadic form of the disease, which accounts for 90% of cases, remains poorly understood. The pathogenesis of ALS is not fully understood in the vast majority of cases, and the mechanisms involved in motor neuron degeneration are multifactorial and complex. There is substantial evidence to support the hypothesis that oxidative stress can underlie motor neuron death.29 Mitochondrial dysfunction and neuroinflammation have also been implicated in ALS pathogenesis. Peroxisome proliferator-activated receptors (PPARs), and in particular PPAR-γ, may form part of a major signaling pathway involved in neuroinflammation in ALS.30 The activation or inactivation of PPAR-γ could provide a viable and promising approach to understanding the mechanism of neuroinflammation in ALS. SIRT1 physically interacts with and deacetylates PPAR-γ, coactivator-1α (PGC-1α) at multiple lysine sites, consequently increasing PGC-1α activity. These findings suggest that PPAR-γ is an important regulator of neuroinflammation, and a new potential target for the development of therapeutic strategies for ALS.31
More recent studies have demonstrated that SIRT1 is protective in vitro against the cytotoxic effects of a mutant superoxide dismutase 1 that causes familial ALS.32
It has been demonstrated that CR is one of the most effective means of slowing the pace of aging and extending lifespan in many organisms, from yeast to mammals. In yeast, the longevity gene induced by CR is Sir2. In mammals, SIRT1, an ortholog of Sir2, controls the metabolism of white adipose tissue. Resveratrol, a polyphenolic compound obtained from grapes and red wine, is the most potent natural product activator of SIRT1. Originally identified through the recognition of the French paradox (a phenomenon whereby individuals with high-fat diets have a low incidence of cardiovascular disease due to the regular consumption of red wine), resveratrol has demonstrated therapeutic efficacies in models of cardiovascular, metabolic, inflammatory, and neurodegenerative diseases, and has shown chemopreventative activity.33 A full understanding of the effects of SIRT manipulation in mammals necessitates the design and generation of additional transgenic and knockout mice to facilitate further investigations into SIRT biology. These models will be critical to elucidating the relationship between SIRTs, metabolism, and aging. SIRT-based therapies (i.e., small-molecule SIRT activators) hold great promise as potential therapeutic modalities for age-related conditions, and especially for neurodegenerative diseases.
Figures and Tables
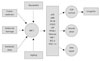 | Fig. 1Convergence of stress signals on SIRT1, an upstream regulator of multiple effectors of the stress response. SIRT: sirtuin, NF-κB: nuclear factor kappa-lightchain-enhancer of activated B cells, FOXO: forkhead transcription factor, LXR: liver X receptor, PPAR: peroxisome proliferator-activated receptor, NBS: nigonegen breakage syndrome, BCL: B-cell lymphoma, PGC: PPAR gamma coactivator. |
Acknowledgements
The author thanks Ms Tracy Ward for critical reading of the manuscript and helpful discussions. This work was supported by the Second-Phase of BK (Brain Korea) 21 Project in 2009.
References
1. Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005. 6:11–22.

2. Guarente L. Sir2 links chromatin silencing, metabolism, and aging. Genes Dev. 2000. 14:1021–1026.

3. Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999. 260:273–279.

4. Oberdoerffer P, Sinclair DA. The role of nuclear architecture in genomic instability and ageing. Nat Rev Mol Cell Biol. 2007. 8:692–702.

5. Westphal CH, Dipp MA, Guarente L. A therapeutic role for sirtuins in diseases of aging? Trends Biochem Sci. 2007. 32:555–560.

6. Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007. 21:1745–1755.

7. Rine J, Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987. 116:9–22.

8. North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003. 11:437–444.

9. Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005. 16:4623–4635.

10. Kennedy BK, Gotta M, Sinclair DA, Mills K, McNabb DS, Murthy M, et al. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell. 1997. 89:381–391.

11. Gan L, Mucke L. Paths of convergence: sirtuins in aging and neurodegeneration. Neuron. 2008. 58:10–14.

12. Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, et al. hSIR2 (SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001. 107:149–159.

13. Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A. 2004. 101:10042–10047.

14. Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004. 303:2011–2015.

15. Sakamoto J, Miura T, Shimamoto K, Horio Y. Predominant expression of Sir2alpha, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett. 2004. 556:281–286.

16. Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003. 100:10794–10799.

17. Wu A, Ying Z, Gomez-Pinilla F. Oxidative stress modulates Sir2alpha in rat hippocampus and cerebral cortex. Eur J Neurosci. 2006. 23:2573–2580.
18. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008. 105:3374–3379.

19. Perrod S, Cockell MM, Laroche T, Renauld H, Ducrest AL, Bonnard C, et al. A cytosolic NAD-dependent deacetylase, Hst2p, can modulate nucleolar and telomeric silencing in yeast. EMBO J. 2001. 20:197–209.

20. Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science. 2007. 317:516–519.

21. Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. 2003. 23:3173–3185.

22. Li W, Zhang B, Tang J, Cao Q, Wu Y, Wu C, et al. Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci. 2007. 27:2606–2616.

23. Valerio A, Boroni F, Benarese M, Sarnico I, Ghisi V, Bresciani LG, et al. NF-kappaB pathway: a target for preventing beta-amyloid (Abeta)-induced neuronal damage and Abeta42 production. Eur J Neurosci. 2006. 23:1711–1720.

24. Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004. 23:2369–2380.

25. Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, et al. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer's disease. FASEB J. 2005. 19:659–661.
26. Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, et al. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol Aging. 2005. 26:995–1000.

28. Okawara M, Katsuki K, Kurimoto E, Shibata H, Kume T, Akaike A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol. 2007. 73:550–560.

29. Barber SC, Mead RJ, Shaw PJ. Oxidative stress in ALS: a mechanism of neurodegeneration and a therapeutic target. Biochim Biophys Acta. 2006. 1762:1051–1067.

30. Shibata N, Kawaguchi-Niida M, Yamamoto T, Toi S, Hirano A, Kobayashi M. Effects of the PPARgamma activator pioglitazone on p38 MAP kinase and IkappaBalpha in the spinal cord of a transgenic mouse model of amyotrophic lateral sclerosis. Neuropathology. 2008. 28:387–398.

31. Kiaei M. Peroxisome Proliferator-Activated Receptor-gamma in Amyotrophic Lateral Sclerosis and Huntington's Disease. PPAR Res. 2008. 2008:418765.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download