Abstract
Background
The medial vestibulospinal tract (MVST), which descends in the medial longitudinal fasciculus (MLF), may mediate the vestibular evoked myogenic potentials (VEMPs) in the contracting sternocleidomastoid muscle. We report herein abnormal VEMPs in a patient with medial medullary infarction (MMI) that appeared to involve the MLF.
Case Report
A patient with infarction involving the right medial medulla showed decreased p13-n23 amplitude and increased p13/n23 latencies of the VEMPs on the right side. These abnormal VEMPs recorded in an MMI patient support the theory that VEMPs are mediated by the MVST contained within the MLF.
The term "vestibular-evoked myogenic potentials (VEMPs)" refers to inhibitory potentials recorded in the contracting muscles, usually the sternocleidomastoid muscles (SCM), when sound stimuli are applied.1-4 The main pathway for saccule-induced inhibitory postsynaptic potentials to ipsilateral SCM motoneurons appears to be the medial vestibulospinal tract (MVST), which descends within the medial longitudinal fasciculus (MLF).5 However, VEMPs have not been measured in lesions involving the medullary MLF. We report herein abnormal VEMPs in a patient with medial medullary infarction (MMI) that appeared to involve the MLF.
A 67-year-old man developed sudden vertigo and vomiting followed by dysarthria and left-sided weakness 9 days before admission to hospital. He had a history of diabetes, hypertension, and chronic renal failure. On admission, he had a blood pressure of 153/79 mmHg, a pulse rate of 75 beats/min, a respiration rate of 20 breaths/min, and a body temperature of 37.0℃. Neurological examination revealed gaze-evoked nystagmus (GEN) during both horizontal and upward gazes. Both eyes deviated to the left during eye closure, and saccades were hypermetric to the left and hypometric to the right. This patient also exhibited dysarthria, dysphagia, and Medical Research Council grade I weakness on the left side. Position and vibration sensations were also decreased to 50% on the left side. The deep tendon reflexes were hypoactive in the extremities, but the toe sign was extensor in the left foot. Other findings of the neurological examination were normal.
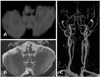
Laboratory tests were notable for increased serum glucose (279 mg/dL, normal range=70-110 mg/dL), hemoglobin A1c (7.7%, normal range=4.0-6.4%), blood urea nitrogen (71 mg/dL, normal range=10-26 mg/dL), creatinine (2.9 mg/dL, normal range=0.7-1.4 mg/dL), and uric acid (8.8 mg/dL, normal range=3.0-7.0 mg/dL). The results of bithermal caloric tests and measurements of ocular torsion and tilt of the subjective visual vertical (SVV) were all normal. Pure-tone audiometry showed symmetric sloping in the lower and higher frequency ranges. VEMPs exhibited decreased p13-n23 amplitude and increased p13/n23 latencies on the right side (Fig. 1). MRI documented a right MMI extending from the pyramid to the medullary tegmentum (Fig. 2A and B). Magnetic resonance (MR) angiography disclosed diffuse narrowing of the right distal vertebral artery and focal narrowings of the left vertebral artery (Fig. 2C).
Our patient, who had an MMI on the right side, showed decreased VEMP p13-n23 amplitude and increased p13/n23 latencies on the right side in addition to GEN, saccadic contrapulsion, dysarthria, hemiparesis, and hemihypesthesia, which are characteristic findings of MMI.6 Since the MLF contains the MVST, which is known to mediate VEMPs recorded in the contracting SCMs, the abnormal VEMPs recorded in our patient with MMI indicate disruption of the MLF in the medullary tegmentum where the MLF is located.
VEMPs can be used to assess the sacculocollic reflex pathway, which travels downward in the lower brainstem.7 The saccule, which is a linear accelerometer in mammals, is an acoustic receptor in lower species, and the vestibular neurons sensitive to click sounds are of otolithic origin.5,8 In peripheral vestibulopathy, abnormal VEMPs are usually associated with canal paresis and the ocular tilt reaction (OTR).4 However, recordings of VEMPs in our patient with MMI disclosed decreased p13-n23 amplitude and increased p13/n23 latency on the ipsilesional side in the absence of caloric paresis, OTR, or SVV tilt. The principal brainstem areas of saccular nerve termination are the inferior vestibular nucleus and the lateral portion of the superior vestibular nucleus, while the principal projections from the utricle are to the laterodorsal medial vestibular nucleus, the ventrolateral superior vestibular nucleus, and the rostral portion of the inferior vestibular nucleus.9 The dissociation of abnormalities between the VEMPs and OTR/SVV tilt suggests differential involvement of the otolithic pathways in central vestibulopathies.
In primates, saccule projects to the superior and inferior vestibular nuclei,9 and proceed bilaterally to the spinal cord. However, sound-evoked VEMPs recorded from the neck are almost completely unilateral.2,4,10 Since the MVST contains inhibitory fibers, the sacculocollic inhibitory signal may travel down from the inferior vestibular nucleus mostly along the MVST to the ipsilateral SCM,2,10-12 while the lateral vestibulospinal tract is excitatory. However, since the MVST originates mostly from the medial vestibular nucleus, the inhibitory sacculocollic responses may also be mediated by the medial vestibular nucleus.13,14 Because the MLF is supplied by the anteromedial medullary arteries and is usually involved in MMI,6 damage to the MLF is most likely to be responsible for the abnormal VEMPs recorded in our MMI patient. This interpretation is also consistent with the associated GEN and the lesion observed on MRI extending to the medullary tegmentum, where the MLF is located. GEN in MMI is ascribed to damage to the nucleus prepositus hypoglossi (NPH) or cell groups of the paramedian tracts, which are also located in the medullary tegmentum.6
In conclusion, the recording of abnormal VEMPs in our MMI patient supports the theory that these potentials are mediated by the MVST contained within the MLF. The association between abnormal VEMPs and GEN also indicates that the lesion in our patient involved the medullary tegmentum where the MLF and the NPH are located. VEMPs may represent a valuable tool for investigating vestibular dysfunction originating from the saccule, even in patients with central vestibulopathies, which is not readily defined by conventional vestibular function tests.
Figures and Tables
Fig. 1
Vestibular evoked myogenic potentials (VEMPs) recorded from the contracting ipsilateral sternocleidomastoid muscle exhibit decreased p13-n23 amplitude and increased p13/n23 latencies on the right side. The stimuli were short, alternating tone bursts (95 dB nHL, 108 dB SPL; 500 Hz; ramp=2 ms; plateau=3 ms) presented at 2.1 Hz monaurally. To compare the normalized p13-n23 amplitude of VEMP responses on the affected side with those on the intact side, the interaural difference ratio (IAD) of the normalized amplitude (IADamp, %) was calculated using [(Au-Aa)/(Aa+Au)×100], where Au is the normalized p13-n23 amplitude on the unaffected side and Aa is the amplitude on the affected side. Normative data were obtained from 52 healthy volunteers (23 women) aged between 22 and 76 years (36.7±2.4 years, mean±SD; median=32 years). Lt: left, Rt: right.

Fig. 2
Brain MRI of the patient with medial medullary infarction. A: Diffusion-weighted axial image disclosing acute infarction in the right medial medulla, which extends from the pyramid to the medullary tegmentum. B: Follow-up T2-weighted axial image also shows infarction in the corresponding area of the acute infarction shown on A. C: MR angiography revealing diffuse narrowing of the right distal vertebral artery (arrow) and focal narrowings in the left vertebral artery (arrowheads). MR: magnetic resonance.
Acknowledgments
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A080750).
The authors thank Jong-Hee Lee for experimental assistance.
References
1. Colebatch JG, Halmagyi GM. Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology. 1992. 42:1635–1636.

2. Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994. 57:190–197.

3. Murofushi T, Matsuzaki M, Wu CH. Short tone burst-evoked myogenic potentials on the sternocleidomastoid muscle: are these potentials also of vestibular origin? Arch Otolaryngol Head Neck Surg. 1999. 125:660–664.

4. Welgampola MS, Colebatch JG. Characteristics and clinical applications of vestibular-evoked myogenic potentials. Neurology. 2005. 64:1682–1688.

5. Murofushi T, Curthoys IS, Topple AN, Colebatch JG, Halmagyi GM. Responses of guinea pig primary vestibular neurons to clicks. Exp Brain Res. 1995. 103:174–178.

6. Kim JS, Choi KD, Oh SY, Park SH, Han MK, Yoon BW, et al. Medial medullary infarction: abnormal ocular motor findings. Neurology. 2005. 65:1294–1298.

7. Chen CH, Young YH. Vestibular evoked myogenic potentials in brainstem stroke. Laryngoscope. 2003. 113:990–993.

8. Fernández C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol. 1976. 39:970–984.

9. Newlands SD, Vrabec JT, Purcell IM, Stewart CM, Zimmerman BE, Perachio AA. Central projections of the saccular and utricular nerves in macaques. J Comp Neurol. 2003. 466:31–47.

10. Murofushi T, Halmagyi GM, Yavor RA, Colebatch JG. Absent vestibular evoked myogenic potentials in vestibular neurolabyrinthitis. An indicator of inferior vestibular nerve involvement? Arch Otolaryngol Head Neck Surg. 1996. 122:845–848.

11. Robertson DD, Ireland DJ. Vestibular evoked myogenic potentials. J Otolaryngol. 1995. 24:3–8.
12. Wilson VJ, Boyle R, Fukushima K, Rose PK, Shinoda Y, Sugiuchi Y, et al. The vestibulocollic reflex. J Vestib Res. 1995. 5:147–170.

13. Pompeiano O, Brodal A. The origin of vestibulospinal fibres in the cat. An experimental-anatomical study with comments on the descending medial longitudinal fasciculus. Arch Ital Biol. 1957. 95:166–195.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download