Abstract
Parkinson's disease (PD) and multiple system atrophy (MSA) are neurodegenerative diseases representative of α-synucleinopathies characterized pathologically by α-synuclein-abundant Lewy bodies and glial cytoplasmic inclusions, respectively. Embryonic stem cells, fetal mesencephalic neurons, and neural stem cells have been introduced as restorative strategies in PD animals and patients, but ethical and immunological problems as well as the serious side effects of tumorigenesis and disabling dyskinesia have limited clinical application of these stem cells. Meanwhile, cell therapy using mesenchymal stem cells (MSCs) is attractive clinically because these cells are free from ethical and immunological problems. MSCs are present in adult bone marrow and represent <0.01% of all nucleated bone marrow cells. MSCs are themselves capable of multipotency, differentiating under appropriate conditions into chondrocytes, skeletal myocytes, and neurons. According to recent studies, the neuroprotective effect of MSCs is mediated by their ability to produce various trophic factors that contribute to functional recovery, neuronal cell survival, and stimulation of endogenous regeneration and by immunoregulatory properties that not only inhibit nearly all cells participating in the immune response cell-cell-contact-dependent mechanism, but also release various soluble factors associated with immunosuppressive activity. However, the use of MSCs as neuroprotectives in PD and MSA has seldom been studied. Here we comprehensively review recent advances in the therapeutic roles of MSCs in PD and MSA, especially focusing on their neuroprotective properties and use in disease-modifying therapeutic strategies.
Parkinson's disease (PD) is a chronic neurodegenerative disease characterized by the selective loss of dopaminergic neurons and the presence of Lewy bodies, which are proteinaceous inclusions that contain α-synuclein, synphilin-1, components of the ubiquitin proteasomal pathway, and parkin in the substantia nigra (SN).1 Recent studies have suggested that the pathogenesis of neuronal degeneration in PD involves several molecular and cellular events, including oxidative stress, proapoptotic mechanisms, mitochondrial dysfunction, and the accumulation of toxic proteins resulting from dysfunction of the protein degradation system.1,2 This results in the emergence of parkinsonian motor symptoms such as bradykinesia, rigidity, tremor, or postural instability when 70% of dopaminergic neurons in the SN are lost. PD is the only chronic neurodegenerative disease for which there are effective symptomatic treatments involving dopaminergic regimens. Nevertheless, these therapies do not change the progressive nature of PD and, moreover, they are ineffective against some axial symptoms of gait freezing and postural instability, which are more disabling than tremors and rigidity.3 In addition, as PD progresses, dopaminergic therapies result in disabling drug-induced motor complications, such as "wearing off" and dyskinesia.
As alternatives for the treatment of PD, cell therapies with dopamine-producing cells that replace dying dopaminergic cells with permanent dopaminergic neurons have been widely considered for future therapies, and PD has been regarded as the best candidate neurodegenerative disease for cell therapy for following reasons: 1) PD is a unique disease involving the selective destruction of dopaminergic neurons in the SN, 2) nigral dopaminergic neurons primarily modulate striatal function and provide tonic stimulation of target receptors, and 3) downstream basal ganglia neurons are relatively preserved.4 Thus, numerous sources of dopamine-generating cell treatments (e.g., embryonic stem cells, fetal mesencephalic neurons, and neural stem cells) have been developed and tested in animal models of PD as well as in patients with PD. Of those, transplantation of embryonic mesencephalic neurons in PD was clinically initiated in 1987 and -350 patients with PD underwent operations.5 In spite of the long-term survival of transplanted cells with clinical and radiological improvement after transplantation in open-label trials, the results of two double-blind controlled trials were not encouraging, and cell transplantation remains currently far from an optimal therapeutic strategy.6,7
Studies of PD have traditionally focused on motor symptoms such as bradykinesia, tremor, rigidity, and postural instability, which originate from the loss of dopaminergic neurons in the SN of the midbrain. However, recent clinical and pathological studies have demonstrated that PD is a complex disease involving multiple systems including the nigral motor system, which is mediated by dopaminergic neuronal loss, as well as extranigral non-motor systems by non-dopaminergic neuronal loss. The olfactory, autonomic, sleep, and cognitive systems are representative affected non-motor systems in PD.8 Based on recent pathoanatomical studies, Braak suggested that the chronological process of PD pathology. According to his staging system,9,10 2 main induction sites of PD pathology exist: the olfactory bulb or anterior olfactory nucleus and enteric nerve cell plexus. Form these sites, PD pathology tends to evolve to other rostral brain areas in a bottom-up fashion, with parkinsonian motor symptoms emerging at stage 3, when the disease involves the midbrain. Thus, α-synuclein accumulation in these non-motor systems-except for the effects on the cognitive system, which manifest in the final stage-precede nigral dopaminergic neuronal pathology, and the functional losses of these nonmotor systems (especially olfactory and cardiac sympathetic systems) appear to be closely coupled and are independent of the clinical nigral motor status.11 Most importantly, these non-motor symptoms are as disabling as nigral motor symptoms.3 Furthermore, PD dementia, which is known to be mediated by cholinergic depletion and thus not to be responsive to dopaminergic therapy, is the most important factor influencing the daily activities and survival of PD patients.
It has been suggested that differences in patient selection, immunosuppression protocols, and tissue storage and preparation may strongly influence the negative outcomes of the large placebo-controlled studies.12 However, most clinical concerns have focused on the development of graft-induced dyskinesia. Freed et al.6 reported that 15% of their grafted patients developed severe postoperative dyskinesias in the "off" state. Hagell et al.13 found that 6 of 14 PD patients with grafted mesencephalic stem cells displayed postoperative "off"-phase dyskinesias of moderate severity. Olanow et al.7 found that 56.5% of grafted patients developed postoperative "off"-state dyskinesias showing stereotypic, rhythmic movements in the lower extremities, which is phenomenologically different from L-dopa-induced peak-dose dyskinesia.
Uneven graft-derived dopaminergic innervation of the striatum,13 inflammation around the implants,14,15 the presence of different subpopulations of dopaminergic neurons in the graft,16 and inappropriate synaptic contacts14 are several possible explanations for graft-induced dyskinesia. However, the exact mechanism and strategies to avoid dyskinesia are still speculative.
Three independent laboratories recently reported autopsy findings from eight subjects who received fetal mesencephalic neurons transplantation. Of those, Kordower et al.17 reported two subjects with PD who exhibited the long-term survival of transplanted fetal mesencephalic dopaminergic neurons (11-16 years) that developed α-synuclein-positive Lewy bodies in grafted neurons. Li et al.18 reported that 14 years after grafted nigral neurons were transplanted into the striatum of an individual with PD, they were found to have Lewy body-like inclusions that stained positively for α-synuclein and ubiquitin and exhibited reduced immunostaining for dopamine transporters. However, Mendez et al.19 reported no PD pathology in grafted neurons in a postmortem analysis of five subjects with PD at 9-14 years after the transplantation of fetal midbrain cell suspensions. Although these case studies have produced conflicting results, the data suggest that the PD microenvironment is prone to ongoing neuronal death and can affect grafted cells in hosts with a similar pathogenesis as in the host dopaminergic neurons, thus leading to pathology propagation from host to graft. The clinical relevance of this-in terms of the long-term clinical efficacy, the frequency that grafted dopaminergic neurons undergo PD pathology, and contributing factors-has remained unclear. Nevertheless, it is possible that newly generated PD pathology in grafted dopaminergic neurons exert detrimental effects on the function of grafted dopaminergic and limit the long-term clinical efficacy of dopaminergic replacement therapy. Along with complex PD etiopathogenesis mechanisms, the nature of PD involving the extranigral systems and the clinical problems with dopaminergic replacement therapy are important issues to be resolved for future clinical cell-based therapies involving dopaminergic replacement.
Mesenchymal stem cells (MSCs) present in adult bone marrow normally provide structure and functional support for hemopoiesis, and they represent <0.01% of all nucleated bone marrow cells. Mesenchymal cells are primordial cells of mesodermal origin that are capable of multipotency, differentiating under appropriate conditions into chondrocytes, skeletal myocytes, and neurons.20,21 Because they exhibit diverse characteristics and consist of a heterogeneous cell population, their true nature is not fully known. Most MSC populations express mesenchymal markers such as CD29 (β-1 integrin), CD90 (Thy-1), CD54 (intracellular adhesion molecule), CD44 (homing-associated cell adhesion molecule), CD71 (transferrin receptor), CD105 (SH2), SH3, Stro-1, and CD13, but they do not express markers typical of hematopoietic and endothelial cell lineages, such as CD11b, CD14, CD 31, CD33, CD34, CD 133, and CD45.22 Cell therapy with MSCs has advantages in clinical applications. MSCs can be easily harvested from the bone marrow of the patient, easily expanded on a large scale for autotransplantation, and administered to patients via various routes, including intravenous, intra-arterial, intrathecal, or intralesional infusion. Additionally, cell therapy with MSCs is free from ethical and immunological problems, which contrasts with embryonic stem cell therapy.
Neurotrophic factors (NTFs) are essential for neuronal survival and differentiation and hence also to the development and maintenance of normal neuronal function in adults. NTFs include several families of structurally and functionally related molecules: the nerve growth factor (NGF) superfamily that comprises two structurally related proteins, namely the brain-derived neurotrophic factor (BDNF) and NGF3; the glial-cell-line-derived neurotrophic factor (GDNF) family; the neurokine superfamily; and the nonneuronal growth factor superfamily. The NTFs relevant to PD are GDNF, BDNF, and neurturin. According to experimental studies using neurotoxin-induced PD models, NTFs slow the progression of degeneration, enhance the activity of remaining neurons, induce regeneration, support the survival of transplanted dopaminergic cells, and induce proliferation and differentiation of neural stem cells.23 Thus, the effectiveness of NTFs in protecting or restoring dopaminergic neurons increases the possibility of applying them clinically as a neuroprotective therapy. However, open-label and double-blinded clinical trials with GDNF in PD have been controversial because of debate regarding the GDNF dose and delivery methods.
There is ample evidence that MSCs produce a variety of NTFs and lead to increased neuronal survival, endogenous cell proliferation, and nerve fiber regeneration.24,25 Crigler et al.26 demonstrated that MSCs express BDNF and β-NGF, and Arnhold et al.27 showed that naive MSCs cultivated in a standard medium express BDNF, NGF, and GDNF. Therefore, the synthesis and release of NTFs relevant to PD by transplanted MSCs or indirect stimulation of neurotrophic release from host tissue might partly contribute to functional recovery, neuronal cell survival, and stimulation of endogenous regeneration after MSC transplantation.28
Recent human and animal studies demonstrated that a glial reaction and inflammatory processes may participate in a cascade of neuronal degeneration in PD. A postmortem study described extensive proliferation of reactive amoeboid microglia in the SN of PD patients,29 suggesting that activating microglia induces dopaminergic neurodegeneration. Another pathological study demonstrated the presence of activated microglia in the SN of PD patients exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP),30 which suggests that an ongoing stimulus can lead to disease progression long after the initial toxic insult. A positron-emission-tomography (PET) study using a radiotracer for activated microglia revealed that microglial activation occurred in patients with early PD and was closely linked to the degree of dopaminergic neuronal loss.31 Furthermore, increased levels of cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and interferon-γ have been demonstrated in the SN of PD patients.32,33 Evidence of inflammation in dopaminergic neuronal death has also been documented in animal models of PD induced by numerous neurotoxins such as MPTP, 6-hydroxydopamine, and rotenone.34-36
Several in vitro and in vivo studies have shown that MSCs possess immunoregulatory properties. Although the exact underlying mechanism is unclear, in vitro studies suggest that MSCs can not only inhibit nearly all cells participating in the immune response cell-cell-contact-dependent mechanism, but can also release various soluble factors that might be involved in the immunosuppressive activity of MSCs.37-39 Recent animal studies in an experimental autoimmune encephalomyelitis demonstrated that MSC treatment results in a significantly milder disease and fewer relapses compared to control animals, with a decreased number of inflammatory infiltrates and reduced demyelination and axonal loss.40,41 Additionally, Guo et al.42 reported that MSC transplantation decreases the protein production and gene expression of inflammation cytokines and increases functional recovery from myocardial infarcts. These studies suggest that the anti-inflammatory action of MSCs is one of the mechanisms underlying the tissue-protective effects.
There is evidence from a large body of animal studies that inhibition of the inflammatory response prevents the degeneration of nigrostriatal dopaminergic neurons. For example, sodium salicylate and cyclooxygenase-2 inhibitors have been shown to significantly reduce dopaminergic neuronal loss induced by MPTP or lipopolysaccharide (LPS).43,44 Additionally, recent epidemiological studies have shown the beneficial effects of nonsteroidal anti-inflammatory drugs (NSAIDs) in the development and progression of PD.45,46 Thus, these studies raise the possibility that the inhibition of inflammation is a viable neuroprotective treatment strategy for PD patients.
MSCs characteristically migrate toward damaged tissues in animal models of ischemia as well as in the PD model induced by 6-hydroxydopamine, possibly in response to signals that are up-regulated under injury conditions.47,48 Although the signals that guide MSCs to the damaged brain are unknown, they might involve chemokines released from the damaged brain and their receptors. Recent studies have demonstrated that stromal-cell-derived factor-1 (SDF-1α) and its receptor CXCR4 play an important role in homing MSCs to ischemic brain lesions.49,50 SDF-1α is widely expressed in brain regions such as the cortex, cerebellum, and globus pallidus, as well as in the SN pars compacta.51 Therefore, as in ischemic brain lesions, it is speculated that damage in the nigrostriatal system of PD animal models induced by neurotoxins increases the expressions of SDF-1α and CXCR4, leading to the recruitment of MSCs to the SN. The number of transplanted MSCs that migrate to the nigral area is unknown in PD animal models, but such migrated cells might contribute to the production of trophic factors and inhibit microenvironmental cascades of the neurodegenerative process in nigral dopaminergic neurons.
Many candidates for neuroprotective agents have been tested in PD animal models. However, most drug candidates have been tested in acute or subacute neurotoxin-induced animal models, with the compounds administered before the development of PD. To replicate clinical conditions as closely as possible when testing neuroprotective agents in a PD animal model, the model should have the same chronic, progressive nature, and the administration of candidate drugs should start after neuronal loss in the SN has started.52 Chronic progressive PD animal models using the systemic injection of proteasome inhibitors are interesting in this regard,53 but there has been extensive debate concerning the outcome of the nigral neuronal loss as well as unidentified factors that are responsible for the observed discrepancies in the results obtained, such as variation in the properties of proteasome inhibitors, environmental factors, and differences in dosing and bioavailability of the toxin in the brain.54-58
We recently evaluated whether MSCs exerted a protective effect on progressive dopaminergic neuronal loss in vitro and in vivo using MG-132, which is a nonspecific proteasome inhibitor (Fig. 1).59 Treating dopaminergic neurons in primary mesencephalic cultures with MSCs for 24 h significantly decreased dopaminergic neuronal loss induced by a 2-h administration of MG-132, and also significantly reduced caspase-3 activity. In rats that received systemic injections of MG-132, there was a progressive decline in the number of tyrosine-hydroxylase-immunoreactive (TH-ir) cells, which was more prominent in the lateral than in the medial regions of the SN. MSC treatment of MG-132-treated rats dramatically increased TH-ir cell survival in the SN, by approximately 50%. Furthermore, MSC treatment markedly decreased the accumulation of polyubiquitinated proteins and caspase-3 activity following MG-132 treatment as well as significantly reduced microglial activation in MG-132-treated animals.
Accordingly, the neuroprotective mechanism of MSC in this progressive experimental model of PD appears to be more complex and pleiotropic, which might be mediated via the modulation of apoptosis, ubiquitin-proteasome function, and microglia activation. Although the initial triggering events of dopaminergic neuronal death in PD remain unknown, apoptosis and altered proteasome activity could play pivotal roles in the pathogenesis of PD. The presence of apoptosis-mediated dopaminergic neuronal death is suggested by DNA fragmentation and chromatin clumping in dopaminergic neurons coupled with up-regulation of signals associated with apoptosis in PD patients.60 Recent genetic, postmortem, and experimental studies have also suggested that proteasomal dysfunction plays an important role in the accumulation of toxic proteins and, consequently, neurodegeneration in the SN, which is mediated by an imbalance between the degradation and clearance of abnormal proteins.61 Additionally, microglial activation appears to occur before the death of dopaminergic neurons, and activated microglia continue to promote degeneration of dopaminergic neurons,34 with the degree of dopaminergic cell loss possibly paralleling the microglial response.35
There is evidence from experimental studies of ischemic heart disease and autoimmune diseases that MSCs exert tissue-protective effects via anti-inflammatory actions,40-42 and we have used LPS-induced in vitro and in vivo inflammation models to investigate whether MSCs exert protective effects on the dopaminergic system via an anti-inflammatory mechanism (Fig. 2).62 In coculture experiments using a Transwell culture chamber system to physically separate LPS-stimulated microglia and MSCs in order to inhibit cell-cell contact, we found that MSCs decreased the number of activated forms of microglia and increased the expressions of the anti-inflammatory cytokines IL-6, IL-10, and transforming growth factor β (TGF-β). Furthermore, MSCs decreased the production of TNF-α and inducible nitroc oxide synthase (iNOS) from microglia stimulated by LPS in a contact-independent manner. In cocultures of microglia and mesencephalic neurons, the anti-inflammatory actions of MSCs actually resulted in a significant decrease (up to -50%) in dopaminergic neuronal death induced by LPS stimulation. Furthermore, an in vivo study found that MSC administration dramatically decreased dopaminergic neuronal loss in the SN induced by LPS stimulation and MPTP treatment, which was clearly accompanied by attenuation of microglial activation, as well as TNF-α and iNOS mRNA expressions and the production of TNF-α. These data show that the neuroprotective effects of MSCs on dopaminergic neurons act via an anti-inflammatory mechanism mediated by the modulation of microglial activation, thus confirming similar observations in animal models of PD.
Recent studies have indicated that human MSCs can differentiate into neuron-like cells.20,21,63 Moreover, Blondheim et al.64 demonstrated that MSCs can express several specific neuronal markers and transcriptional factors, with a large proportion of the genes participating in the neuro-dopaminergic system. Barzilay et al.65 recently reported a serum-free controlled differentiation protocol that yielded dopamine-producing cells from MSCs, with more than 30% of the cells expressing significant levels of TH. There have been a few reports of MSCs differentiating into TH-ir neurons in in vivo studies, but the results were contradictory. Li et al.48 reported that MSCs injected intrastriatally exhibited the phenotype of dopaminergic neurons in MPTP animal models, and Blondheim et al.64 and Offen et al.66 demonstrated that intrastriatal transplantation of undifferentiated and differentiated MSCs in 6-hydroxydopamine-induced animal models led to the expression of TH in the striatum. However, Ye et al.67 did not find BrdU and TH-ir cells in the striatum, and suggested that functional recovery in MSC-treated rats is not associated with differentiation of MSCs into TH-ir neurons. We found that approximately 35.7% of surviving MSCs in the SN displayed TH immunoreactivity in progressive PD animal models and that TH-ir cells could be immunostained with human-specific synaptophysin, suggesting that TH-ir cells have a dopaminergic function. However, there are no data confirming the transdifferentiation of MSCs into functional dopaminergic neurons.
Multiple system atrophy (MSA) is a sporadic, progressive, adult-onset neurodegenerative disorder associated with varying degrees of parkinsonism, autonomic dysfunction, and cerebellar ataxia, and is characterized pathologically by widespread α-synuclein-positive glial cytoplasmic inclusions in the brain and spinal cord.68 Disease progression is much faster in MSA than in PD, and there is no drug treatment that provides MSA patients with consistent long-term benefits. Therefore, neuroprotective or regenerative strategies are required for managing MSA patients. We observed the long-term clinical and radiological effects of MSCs in patients with MSA. In an open-label study design, the neurological deficits in 11 patients with MSA who received consecutively intra-arterial and 3 repeated intravenous injections for 3 months were compared with 18 nontreated MSA patients.69 The improvement in neurological deficits as measured on the Unified MSA Rating Scale was significantly greater in MSC-treated patients than in the control patients at all visits throughout the 12-month study period (Fig. 3A and B). Serial PET scans performed on subgroups revealed that cerebral glucose metabolism in the follow-up scans of MSC-treated patients was increased significantly in the cerebellum and frontal white matter, whereas cerebral glucose metabolism in the follow-up scans of the control group decreased significantly in the cerebellum and brainstem (Fig. 3C and D). There were no serious adverse effects related to MSC therapy. Although the study is limited by its open-label trial design, with a double-blind placebo trial being required to resolve remaining controversies,70,71 it does provide clinical clues to the neuroprotective properties of MSCs in MSA.
There is ample evidence that MSCs exert neuroprotective effects against dopaminergic neuronal death. Complex mechanisms might underlie the functional recovery from PD by homing MSCs into the SN, modulation of apoptosis, ubiquitin-proteasome function, and immunomodulation from migrated cells, thus inhibiting microenvironmental cascades of the neurodegenerative process in nigral dopaminergic neurons. The advantages of MSCs in clinical applications could mean that their neuroprotective properties have major therapeutic implications as candidate disease-modifying strategies for PD and MSA.
Figures and Tables
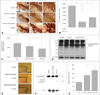 | Fig. 1Effects of cell therapy with human mesenchymal stem cells (hMSCs) on animals treated with MG-132. Immunohistochemical analysis showed that hMSC treatment dramatically reduced the decline in the number of TH-ir cells in the SN of MG-132-treated rats (A). Stereological analysis revealed that the number of TH-ir cells was significantly higher in the hMSC-treatment group than in the group treated with MG-132 alone (n=5; p<0.05, B). Dopamine levels in the striatum (as assessed by gas chromatography-mass spectrometry) were significantly lower in MG-132-treated rats than in controls (p<0.01); however, hMSC treatment significantly increased the dopamine level in the striatum of MG-132-treated rats (n=5; p<0.05, C). MG-132 treatment resulted in the accumulation of polyubiquitinated proteins and a markedly increase in OX-6 immunoreactivity; however, hMSC treatment markedly decreased the accumulation of polyubiquitinated proteins and OX-6 immunoreactivity in MG-132-treated rats (D and E). The level of the cleaved form of caspase-3 was significantly lower in rats treated with hMSCs (F) than in MG-132-treated rats (n=3, G). Scale bar: 100 µm. *p<0.05, **p<0.01. SN: substantia nigra, TH-ir: tyrosine-hydroxylase-immunoreactive. |
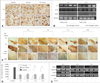 | Fig. 2Coculturing hMSCs with LPS-stimulated microglia in a Transwell culture chamber system decreased microglial activation and increased the expressions of IL-6, IL-10, and TGF-β. To identify soluble factors associated with modulation of microglial activation, we analyzed the expressions of IL-6, IL-10, and TGF-β in hMSCs cocultured with LPS-stimulated microglia and in hMSCs alone. The inclusion of hMSCs significantly decreased the number of process-bearing activated microglia at 6 and 24 h following hMSC treatment (A). When hMSCs were cocultured with LPS-stimulated microglia, IL-6 expression was significantly increased at 3 and 12 h, and the expressions of IL-10 and TGF-β at 12 h were significantly higher than those with hMSCs alone (B). Immunohistological evaluation of protective effect of hMSCs against LPS-induced damage to dopaminergic neurons in the SN. hMSC treatment considerably reduced the loss of TH-ir cells and microglial activation induced by LPS stimulation in the SN (C, Scale bar: 100 mm). Stereological analysis revealed that hMSC treatment significantly decreased the loss of TH-ir cells at 7 and 14 days following LPS stimulation (D, *p<0.05). The administration of hMSCs significantly down-regulated the LPS-induced increase in the expressions of TNF-α and iNOS mRNA at 3 days after LPS stimulation (E). hMSCs: human mesenchymal stem cells, LPS: lipopolysaccharide, IL: interleukin, TGF-β: transforming growth factor β, SN: substantia nigra, TNF-α: tumor necrosis factor-α. |
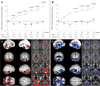 | Fig. 3Changes from baseline scores (mean and SE values) on the Unified Multiple System Atrophy Rating Scale (UMSARS) for MSC-treated and control patients throughout the 12 months of follow-up (A). UMSARS I analysis between MSC-treated and control patients (B: black squares=MSC-treated patients; gray triangles=control patients). The improvement on the UMSARS was significantly greater in the MSC group than in the control group at all visits throughout the 12-month study period. Cerebral glucose metabolism in the MSC-treated patients was higher in the follow-up scan than in the initial scan in the cerebellum and white matter (C, red color), whereas in the control group it was significantly lower in the follow-up scan than in the initial scan in the cerebellum and brainstem (D, blue color). MSC: mesenchymal stem cell. |
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund)(KRF-2008-331-E00305).
References
1. Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005. 28:57–87.

2. von Bohlen und Halbach O, Schober A, Krieglstein K. Genes, proteins, and neurotoxins involved in Parkinson's disease. Prog Neurobiol. 2004. 73:151–177.

4. Palmer MR, Granholm AC, van Horne CG, Giardina KE, Freund RK, Moorhead JW, et al. Intranigral transplantation of solid tissue ventral mesencephalon or striatal grafts induces behavioral recovery in 6-OHDA-lesioned rats. Brain Res. 2001. 890:86–99.

6. Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001. 344:710–719.

7. Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003. 54:403–414.

9. Braak H, Del Tredici K. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology. 2008. 70:1916–1925.

10. Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004. 318:121–134.

11. Lee PH, Yeo SH, Kim HJ, Youm HY. Correlation between cardiac 123I-MIBG and odor identification in patients with Parkinson's disease and multiple system atrophy. Mov Disord. 2006. 21:1975–1977.

12. Correia AS, Anisimov SV, Li JY, Brundin P. Stem cell-based therapy for Parkinson's disease. Ann Med. 2005. 37:487–498.

13. Hagell P, Piccini P, Björklund A, Brundin P, Rehncrona S, Widner H, et al. Dyskinesias following neural transplantation in Parkinson's disease. Nat Neurosci. 2002. 5:627–628.

14. Björklund A, Dunnett SB, Brundin P, Stoessl AJ, Freed CR, Breeze RE, et al. Neural transplantation for the treatment of Parkinson's disease. Lancet Neurol. 2003. 2:437–445.

15. Winkler C, Kirik D, Björklund A. Cell transplantation in Parkinson's disease: how can we make it work? Trends Neurosci. 2005. 28:86–92.

16. Isacson O, Bjorklund LM, Schumacher JM. Toward full restoration of synaptic and terminal function of the dopaminergic system in Parkinson's disease by stem cells. Ann Neurol. 2003. 53:Suppl 3. S135–S146. discussion S146-S148.

17. Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008. 14:504–506.

18. Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008. 14:501–503.

19. Mendez I, Viñuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, et al. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 2008. 14:507–509.

20. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.

21. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.

22. Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood). 2001. 226:507–520.

23. Bonuccelli U, Del Dotto P. New pharmacologic horizons in the treatment of Parkinson disease. Neurology. 2006. 67:S30–S38.

24. Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002. 59:514–523.

25. Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004. 55:1185–1193.

26. Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006. 198:54–64.

27. Arnhold S, Klein H, Klinz FJ, Absenger Y, Schmidt A, Schinköthe T, et al. Human bone marrow stroma cells display certain neural characteristics and integrate in the subventricular compartment after injection into the liquor system. Eur J Cell Biol. 2006. 85:551–565.

28. Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004. 36:568–584.

29. McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988. 38:1285–1291.

30. Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999. 46:598–605.

31. Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, et al. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005. 57:168–175.

32. Hunot S, Dugas N, Faucheux B, Hartmann A, Tardieu M, Debré P, et al. FcepsilonRII/CD23 is expressed in Parkinson's disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. J Neurosci. 1999. 19:3440–3447.

33. Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson's disease. J Neural Transm Suppl. 2000. 143–151.

34. Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002. 22:782–790.

35. Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, Isacson O. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur J Neurosci. 2002. 15:991–998.

36. Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999. 5:1403–1409.

37. Krampera M, Pasini A, Pizzolo G, Cosmi L, Romagnani S, Annunziato F. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol. 2006. 6:435–441.

38. Karussis D, Kassis I, Kurkalli BG, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci. 2008. 265:131–135.

39. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007. 110:3499–3506.

40. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005. 106:1755–1761.

41. Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007. 61:219–227.

42. Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007. 30:97–104.

43. Aubin N, Curet O, Deffois A, Carter C. Aspirin and salicylate protect against MPTP-induced dopamine depletion in mice. J Neurochem. 1998. 71:1635–1642.

44. Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, et al. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci U S A. 2003. 100:5473–5478.

45. Chen H, Zhang SM, Hernán MA, Schwarzschild MA, Willett WC, Colditz GA, et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003. 60:1059–1064.

46. Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology. 2007. 69:1836–1842.

47. Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003. 73:778–786.

48. Li Y, Chen J, Wang L, Zhang L, Lu M, Chopp M. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine mouse model of Parkinson's disease. Neurosci Lett. 2001. 316:67–70.

49. Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, et al. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002. 22:5865–5878.

50. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007. 25:2739–2749.

51. Banisadr G, Skrzydelski D, Kitabgi P, Rosténe W, Parsadaniantz SM. Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. Eur J Neurosci. 2003. 18:1593–1606.

52. Emborg ME. Evaluation of animal models of Parkinson's disease for neuroprotective strategies. J Neurosci Methods. 2004. 139:121–143.

53. McNaught KS, Perl DP, Brownell AL, Olanow CW. Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson's disease. Ann Neurol. 2004. 56:149–162.

54. Schapira AH, Cleeter MW, Muddle JR, Workman JM, Cooper JM, King RH. Proteasomal inhibition causes loss of nigral tyrosine hydroxylase neurons. Ann Neurol. 2006. 60:253–255.

55. Zeng BY, Bukhatwa S, Hikima A, Rose S, Jenner P. Reproducible nigral cell loss after systemic proteasomal inhibitor administration to rats. Ann Neurol. 2006. 60:248–252.

56. Bové J, Zhou C, Jackson-Lewis V, Taylor J, Chu Y, Rideout HJ, et al. Proteasome inhibition and Parkinson's disease modeling. Ann Neurol. 2006. 60:260–264.

57. Kordower JH, Kanaan NM, Chu Y, Suresh Babu R, Stansell J 3rd, Terpstra BT, et al. Failure of proteasome inhibitor administration to provide a model of Parkinson's disease in rats and monkeys. Ann Neurol. 2006. 60:264–268.

58. Manning-Boğ AB, Reaney SH, Chou VP, Johnston LC, McCormack AL, Johnston J, et al. Lack of nigrostriatal pathology in a rat model of proteasome inhibition. Ann Neurol. 2006. 60:256–260.

59. Park HJ, Lee PH, Bang OY, Lee G, Ahn YH. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. J Neurochem. 2008. 107:141–151.

60. Tatton WG, Chalmers-Redman R, Brown D, Tatton N. Apoptosis in Parkinson's disease: signals for neuronal degradation. Ann Neurol. 2003. 53:Suppl 3. S61–S70. discussion S70-S72.

61. Olanow CW, McNaught KS. Ubiquitin-proteasome system and Parkinson's disease. Mov Disord. 2006. 21:1806–1823.

62. Kim YJ, Park HJ, Lee G, Bang OY, Ahn YH, Joe E, et al. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009. 57:13–23.

63. Mareschi K, Novara M, Rustichelli D, Ferrero I, Guido D, Carbone E, et al. Neural differentiation of human mesenchymal stem cells: Evidence for expression of neural markers and eag K+ channel types. Exp Hematol. 2006. 34:1563–1572.

64. Blondheim NR, Levy YS, Ben-Zur T, Burshtein A, Cherlow T, Kan I, et al. Human mesenchymal stem cells express neural genes, suggesting a neural predisposition. Stem Cells Dev. 2006. 15:141–164.

65. Barzilay R, Kan I, Ben-Zur T, Bulvik S, Melamed E, Offen D. Induction of human mesenchymal stem cells into dopamine-producing cells with different differentiation protocols. Stem Cells Dev. 2008. 17:547–554.

66. Offen D, Barhum Y, Levy YS, Burshtein A, Panet H, Cherlow T, et al. Intrastriatal transplantation of mouse bone marrow-derived stem cells improves motor behavior in a mouse model of Parkinson's disease. J Neural Transm Suppl. 2007. 133–143.

67. Ye M, Wang XJ, Zhang YH, Lu GQ, Liang L, Xu JY, et al. Therapeutic effects of differentiated bone marrow stromal cell transplantation on rat models of Parkinson's disease. Parkinsonism Relat Disord. 2007. 13:44–49.

68. Wenning GK, Colosimo C, Geser F, Poewe W. Multiple system atrophy. Lancet Neurol. 2004. 3:93–103.

69. Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008. 83:723–730.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download