Abstract
An anaphylactoid reaction to recombinant tissue plasminogen activator (rt-PA) is an uncommon but fatal complication. A 39-year-old man was admitted within 1 hour of the onset of a right hemispheric stroke. He was not taking any specific medication, including angiotensin-converting enzyme (ACE) inhibitors. A systemic anaphylactoid reaction developed immediately after rt-PA infusion. However, the symptoms were improved after treatment with a steroid and antihistamine. Subsequent intra-arterial thrombolytic therapy resulted in complete recanalization and clinical improvement. To our knowledge, this is the first report of a life-threatening anaphylactoid reaction after rt-PA treatment followed by successful intra-arterial thrombolytic therapy in a patient who had not taken an ACE inhibitor.
Intravenous thrombolysis with recombinant tissue plasminogen activator (rt-PA) is the only approved treatment for use within 3 hours of the onset of acute ischemic stroke. In addition to the well-known symptomatic intracranial hemorrhage, life-threatening orolingual angioedema and anaphylactoid reaction have been reported as serious complications in patients with rt-PA,1-6 and these complications have been emphasized in current treatment guidelines.7
In most cases, previous medication with an angiotensin-converting enzyme (ACE) inhibitor is known to be a preceding factor for the development of orolingual angioedema. Here we report on a life-threatening anaphylactoid reaction after rt-PA treatment followed by successful intra-arterial thrombolysis in a patient without a history of ACE inhibitor use.
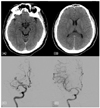
A 39-year-old man was admitted because of a sudden weakness on the left side of his body that had developed 1 hour before admission. One year previously he had experienced a transient ischemic attack involving weakness of the right side of his body lasting for 5 minutes. He had not taken any medication for several months before admission. His medical history was negative for diabetes mellitus and hypertension. A neurological examination indicated that he was alert, but his eyeballs were partially deviated to the right side with left hemianopia and probable visual hemineglect. Left-sided hemiparesis (MRC grade I) with densely decreased sensation was observed. The plantar response was positive on the right side. The score on the National Institutes of Health Stroke Scale (NIHSS) was 17. His blood pressure was 130/90 mmHg and his pulse rate was 88 beats/minute. Electrocardiography showed atrial fibrillation. Complete blood count, serum chemistry, and coagulation parameters including the prothrombin time and activated partial thromboplastin time were normal. Brain CT performed 80 minutes after stroke onset revealed loss of the differentiation between gray- and white-matter effacement of cerebral sulci (Fig. A, B). No other early ischemic changes were evident. Intravenous rt-PA was administered 100 minutes after stroke onset according to NINDS rt-PA criteria8 with the patient receiving a 5.85 mg bolus over 1 minute followed by 52.65 mg over 60 minutes. Fifteen minutes after the infusion commenced, the patient presented with dyspnea followed by a rapid decrease in oxygen saturation of up to 90% and sinus tachycardia, which was accompanied by urticaria spreading from the lower abdomen to the chest, neck, and upper extremities without orolingual angioedema. His blood pressure dropped to 90/40 mmHg and his pulse rate increased to 110 beats/minute. Stridor and wheezing developed, followed by cyanosis, and then the patient descended to a stupor. rt-PA infusion was discontinued, and he was treated with 100 mg hydrocortisone, 8 mg chlorpheniramine, and 50 mg ranitidine, and endotracheal intubation was performed. The vital signs normalized after 10 minutes, and he became alert after 40 minutes. A neurological examination showed improved findings, and he eventually returned to a state similar to that upon admission.
Following this improvement, we performed intra-arterial thrombolysis with urokinase 4 hours after the onset of stroke. A conventional angiogram performed at the same time revealed an occlusion on the proximal portion of the right middle cerebral artery (M1 division). The administration of 80,000 U of urokinase at the occlusion site intra-arterially resulted in complete recanalization (Fig. C, D). The NIHSS score was markedly improved from 17 to 9 at 24 hours after rt-PA treatment.
This is the first reported case of a life-threatening anaphylactoid reaction after rt-PA infusion followed by successful intra-arterial thrombolysis. Since rt-PA was the only substance consumed by the patient that could have induced the anaphylactoid reaction, which occurred immediately after the rt-PA infusion, we consider that rt-PA induced this reaction.
The induction of an anaphylactoid reaction by rt-PA has been reported in less than 2% of patients treated for acute myocardial infarction.9 Anaphylactoid reactions reportedly occurred in between 0.9%5 and 5.1%3 of acute ischemic stroke patients, most of whom had been taking ACE inhibitors. They usually presented with orolingual angioedema with or without other symptoms such as hemodynamic collapse, urticaria, airway obstruction, and shock. rt-PA is an endogenous agent consisting of alteplase (a direct thrombolytic agent) with phosphoric acid and arginine added to adjust the pH and increase solubility. Despite the low antigenic potential of serum, specific IgG and IgM antibodies have been found in serum within days or weeks after administering rt-PA,10 and IgE antibodies were detected in one case of anaphylaxis without angioedema.11
An anaphylactoid reaction is more likely to result from direct activation of the complement system by plasmin. The activated complement pathway subsequently stimulates mast cells and basophils to release potent vasoactive substances, such as bradykinins and histamine, which can be provoked by ACE inhibitors.12 Since we did not measure the immunoglobulin level, the exact underlying mechanism remains unclear.
We did not use epinephrine for the medical management of the anaphylactoid reaction due to concern about possible vasoconstriction of intracranial vessels and hemorrhage with a sudden increase in blood pressure. After the resolution of symptoms, we continued with intra-arterial thrombolysis, which resulted in complete recanalization. Fortunately, the patient did not show adverse effects after administering urokinase, instead showing a gradual clinical improvement. Although combined intravenous and intra-arterial thrombolysis is not yet approved, selective infusion of thrombolytic agents in patients with persisting large-artery occlusion can result in early recanalization, finally leading to an improved functional outcome. Moreover, the safety of intra-arterial thrombolysis after full-dose intravenous rt-PA was recently reported.13 When a patient develops an anaphylactoid reaction after rt-PA and has a persisting large-artery occlusion, it might be possible to proceed to intra-arterial thrombolysis after resolving the anaphylactoid reaction.
In conclusion, the possibility of a life-threatening anaphylactoid reaction after rt-PA treatment should be considered despite this being a rare adverse event. Even after the occurrence of a life-threatening anaphylactoid reaction related to rt-PA infusion, intra-arterial urokinase thrombolysis should be considered in order to obtain a better outcome. The immediate and appropriate management of our patient improved the neurological outcome.
One limitation of this study is that we did not perform tests for identifying the cause of anaphylaxis. However, the clinical presentation was undoubtedly indicative of an anaphylactoid reaction, and hence it was not necessary to perform detailed testing to reach the clinical decision.
Figures and Tables
References
1. Hill MD, Barber PA, Takahashi J, Demchuk AM, Feasby TE, Buchan AM. Anaphylactoid reactions and angioedema during alteplase treatment of acute ischemic stroke. Can Med Assoc J. 2000. 162:1281–1284.
2. Krmpotic KR, Fernandes CM. Anaphylactoid reaction to recombinant tissue plasminogen activator. Eur J Emerg Med. 2007. 14:60–61.

3. Hill MD, Lye T, Moss H, Barber PA, Demchuk AM, Newcommon NJ, et al. Hemi-orolingual angioedema and ACE inhibition after alteplase treatment of stroke. Neurology. 2003. 60:1525–1527.

4. Pancioli A, Brott T, Donaldson B, Miller R. Asymmetric angioneurotic edema associated with thrombolysis for acute stroke. Ann Emerg Med. 1997. 30:227–229.

5. Rudolf J, Grond M, Schmülling S, Neveling M, Heiss W. Orolingual angioneurotic edema following therapy of acute ischemic stroke with alteplase. Neurology. 2000. 55:599–600.

6. Engelter ST, Fluri F, Buitrago-Téllez C, Marsch S, Steck AJ, Ruegg S, et al. Life-threatening orolingual angioedema during thrombolysis in acute ischemic stroke. J Neurol. 2005. 252:1167–1170.

7. Adams HP Jr, del Zoppo G, Albers MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007. 38:1655–1711.

8. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995. 333:1581–1587.
9. Massel D, Gill JB, Cairns JA. Anaphylactoid reaction during an infusion of recombinant tissue-type plasminogen activator for acute myocardial infarction. Can J Cardiol. 1991. 7:298–302.
10. Cugno M, Cicardi M, Colucci M, Bisiani G, Merlini PA, Spinola A, et al. Non neutralizing antibodies to tissue type plasminogen activator in the serum of acute myocardial infarction patients treated with the recombinant protein. Thromb Haemost. 1996. 76:234–238.

11. Rudolf J, Grond M, Prince WS, Schmülling S, Heiss WD. Evidence of anaphylaxy following alteplase infusion. Stroke. 1999. 30:1142–1143.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download