This article has been retracted. See "Retraction" in Volume 8 on page 160.
Abstract
Stroke is associated with high disability and mortality burdens worldwide, but there are few effective and widely available therapies. There is therefore a need to develop treatments that promote the repair and regeneration of ischemic brain tissue. In this regard, a population of adult stem cells-called endothelial progenitor cells (EPCs)-has been identified in peripheral blood that could provide novel approaches in regenerative medicine for curing patients with acute ischemic stroke. There is accumulating evidence that EPCs can repair damaged endothelia and attenuate the development and progression of atherosclerosis. Also, EPCs can be recruited in response to acute ischemic events and participate in reparative vasculogenesis. Most studies related to EPCs have involved patients with cardiovascular diseases, and there is emerging evidence that EPCs represent a risk marker and a potential therapeutic agent in cerebrovascular disease. Here we review the characteristics and biology of EPCs in cerebrovascular disease and discuss the challenges that must be addressed to clarify the role and therapeutic applicability of EPCs in cerebrovascular disease.
Stroke is an important cause of mortality and disability worldwide,1 with an associated high socioeconomic impact, but the only effective curative therapeutic approaches are thrombolytic treatments, which have narrow time windows and limited availabilities. Furthermore, stroke eventually leads to tissue necrosis and possibly to irreversible impairment of brain function.2 Therefore, repair processes after cerebral ischemia should be investigated in order to develop therapeutic strategies for promoting neurorecovery. Endothelial repair and neovascularization are possible in the adult brain recovering from ischemic stroke, and could include both angiogenesis and vasculogenesis-combining these two processes could be one of the most promising therapeutic strategies for stroke.
Endothelial progenitor cells (EPCs) are a type of adult stem cell that has been actively investigated. These immature hematopoietic endothelial cells circulate in peripheral blood (PB).3 EPCs counteract ongoing risk-factor-induced endothelial cell injury, and in response to acute hypoxia are mobilized from bone marrow (BM) to PB and participate in endothelial cell repair and regeneration and also in tissue neovascularization.4 Experimental and human studies have shown that EPCs participate in neovascularization processes in ischemic organs, and hence their regulation could have therapeutic applications in various vascular diseases.5-7
Increased cardiovascular risk factors and the presence of atherosclerosis are associated with dysfunction and reduced numbers of EPCs.4,8-11 Moreover, a low number of EPCs is an independent risk factor for future cardiovascular events.12,13 Coronary artery disease and cerebrovascular disease (CVD) are two sides of the same coin with similar etiologies that result in endothelial damage and arteriosclerosis. Although EPCs have been studied in cardiac disease and might be surrogate markers of vascular function, there have been only a few observation studies on the contribution of EPCs to CVD.14-20 Biological assays of EPCs in stroke might reveal the specific mechanisms of ischemic lesions and predict their severities and outcomes. In this review, we discuss the current developments in EPC research with special emphasis on CVD, including in the risks of stroke, acute stroke, and chronic stroke. We also discuss the challenges of transplanting EPCs as a treatment for stroke.
BM contains a mobile pool of nonhematopoietic cells that express various markers of tissue-committed stem cells that could migrate to the peripheral circulation and exert beneficial effects on regeneration.21-23 Their mobilization, recruitment, and homing mechanisms are regulated by various chemokines and cytokines, and several physiological and pathological conditions can influence the number of circulating progenitor cells. EPCs constitute a population of purified cells that originate directly from the hemangioblast, a common precursor of hematopoietic and endothelial cells.24 Such EPCs most likely coexpress specific endothelial and stem/progenitor markers such as vascular endothelial (VE)-cadherin and CD133, and whilst being extremely rare in PB they are markedly increased after vascular trauma.25 EPCs isolated from PB differentiate into a mature endothelial phenotype based on their microscopic appearance, functional characteristics {uptake of acetylated low-density lipoprotein (acLDL) and nitric oxide synthesis}, and expression of cell-surface markers {E-selectin, von Willebrand factor (vWF), VE-cadherin, platelet-endothelial cell adhesion molecule, and c-kit} associated with loss of CD133 expression.25
BM cells circulating in the PB increase in number during tissue injury and are chemoattracted to the ischemic tissues. It has been shown that BM progenitor cells can be released from BM/tissue niches, circulate, and finally be chemoattracted to ischemic tissue in an SDF-1/CXCR4-dependent manner.26 In a manner characteristic of circulating BM cells, EPCs are mobilized during ischemia or exogenously by stimulation with cytokines, and contribute to neovascularization of ischemic tissues.7,27-29 An experimental study found that the number of CD34+ EPCs gradually increased for 7 days after a stroke and remained significantly above the prestroke baseline to day 14, returning to baseline by day 30.14
EPCs can be isolated from PB in sufficient quantities for harvesting, and after their ex vivo expansion they can be administered systemically to enhance neovascularization. Thus, circulating EPCs have the potential for vascular repair after injury. Vascular progenitor cells have been shown to incorporate into areas of active vascular growth in animal models of hind-limb, myocardial, and cerebral ischemia,7,27 indicating their therapeutic potential either by providing endothelial cells for new vessel growth or through secretion of angiogenic growth factors that activate neighboring cells. The suggested regenerative potential of EPCs has prompted clinical studies of the following two hypotheses: (1) patients with lower numbers of EPCs have a higher risk of vascular diseases, and (2) patients with ischemic events would benefit from EPC transplantation.
EPCs are maturating cells derived from immature stem cells, and hence they possess functional and structural characteristics of both stem cells and mature endothelial cells. During their development, EPCs gradually lose stem-cell characteristics and progressively gain endothelial-cell characteristics. EPCs should be quantified with caution due to their heterogeneity. Two quantification techniques have been reported. The first is flow cytometry, in which cells are labeled with fluorescent antibodies to EPC cell-surface antigens (see Table 1) and subsequently counted with a flow cytometer. The rarity of EPCs in PB makes it necessary to reduce the noise-to-signal ratio.30 Isolated BM-derived EPCs represent more immature cells, expressing the early hematopoietic marker CD133,31 and their phenotype is in general CD133+/CD34+/VEGFR-2+/VE-cadherin-.32 In contrast, EPCs isolated from PB obviously lose CD133 and gradually start to express CD31, VE-cadherin, c-Kit, vWF, CD146, and CXCR4.3,32 A combination of CD34 and VEGR2 cell-surface markers in a mononuclear population has recently been recommended as the best choice for EPC identification.33,34 The second technique is cell culture (see Fig. 1), in which PB-derived mononuclear cells (PBMNCs) are cultured for several days in conditions that selectively favor the growth of EPCs. These conditions include using gelatin-coated plates and adding endothelial growth factors to the culture medium. Since EPCs have to be viable and able to respond to the culture conditions, culture-based quantification of EPCs also depends on their function. EPCs forming colony-forming units (CFU) from the third day after plating are typically counted after 7 to 28 days. Further testing to confirm the endothelial phenotype of the cells involves the uptake of acLDL, binding of Ulex europaeus lectin, and binding of specific antibodies.3,19,20 In contrast to flow cytometry, culturing methods depend on EPC function, and indirectly measure the number of EPCs. This difference means that the results from the two widely used techniques cannot be directly compared,34 making some sort of standardization desirable.
Interpreting the results from the ever-increasing number of reports on EPC biology has been hampered by a lack of corroborated methods for precisely identifying EPCs. Some investigators have used flow cytometry to estimate EPC numbers,8,18,29 whereas others refer to the number of CFU. Since the number of circulating EPCs represents a dynamic balance between their production induced by chronic or acute stimuli and their consumption in damaged areas, performing flow cytometry at a small number of time points during acute ischemia is of limited usefulness. There is emerging evidence that EPC functional properties are better represented by the number of CFU than by the number of EPCs.4,15,17,19,20 Whereas EPC function is critical for endothelial maintenance and repair, the number of CFU might indicate the cumulative vascular risk in ischemia.
Since Asahara et al. first reported the existence of EPCs in PB,3 several studies have found significant heterogeneity among EPC populations in in vitro cultures. At least three types of EPC have been described (Table 1). PBMNCs contain cells termed "early EPCs" that share some endothelial but also monocytic characteristics and a restricted capacity for expansion.35 Recent studies have also shown the existence of a more promising population that originate from BM, circulate in PB, and whose morphology and proliferation pattern differs from the EPCs reported by Asahara et al.3 So-called endothelial outgrowth cells appear after 2 to 3 weeks of culture, rapidly replicate from multiple cells, and form into monolayers with a cobblestone-like morphology and a high proliferation capacity.19,35,36 Current data suggest that this population of outgrowing cells is a subset of true EPCs deriving from BM that exhibit the potential for vascular repair after injury. More recently, we have also isolated and cultured two types of outgrowing cells obtained from the PB of patients with acute stroke.19 These cells were named endothelial or neuronal outgrowth cells (NOCs), according to their morphological characteristics and protein or gene expression profiles. Both types of outgrowing cells maintained their proliferative capacities during a culture period of 3 months. Although numerous reports have described the clinical significance of circulating EPCs, there are few data supporting their stem or progenitor status-namely, the ability to give rise to proliferating, functional endothelial cells such as outgrowing cells. We have performed studies validating the number of outgrowth cells as a potential marker representing the number of circulating progenitor cells.19,20
Circulating EPCs could be a marker of endothelial function and cardiovascular risk. EPC numbers are significantly decreased in subjects with elevated serum cholesterol, hypertension, and diabetes, and in smokers.37,38 Measurements of flow-mediated brachial-artery reactivity also revealed a significant relation between endothelial function and the number of EPCs, supporting a role for EPCs in the maintenance of endothelial integrity.4 Endothelial cell injury and endothelial dysfunction are predictors of the risk of vascular events, providing stimuli for the development of atherosclerotic plaques.8 Consistent with this hypothesis, cardiovascular risk factors-as well as coronary, cerebral, and peripheral atherosclerosis-have been associated with low EPC numbers,4,8-11,15,39 which has also been shown to represent an independent risk factor for future cardiovascular events.12,13 Moreover, depletion of CD34+/KDR+ EPCs was found to be an independent predictor of early subclinical atherosclerosis in healthy subjects.39
There have been a few observation studies on the contribution of EPCs to cerebral atherosclerosis and stroke. Table 2 summarizes data from previous studies related to circulating EPCs in CVD. In patients with prior stroke, the number of circulating CD34+/KDR+ EPCs is negatively correlated with carotid intima-media thickness, and is an independent risk factor for increased carotid intima-media thickness and the presence of carotid plaques.16 Furthermore, stroke patients with lower than normal numbers of CD34+/KDR+ EPCs had a significantly greater carotid intima-media thickness and a significantly higher prevalence of carotid plaques. In addition, there was a strong negative correlation between the numbers of circulating CD34- and CD133-positive cells and the presence of old infarction.14 Analysis of patients with cerebral artery occlusion revealed a significant positive correlation between circulating CD34- and CD133-positive cells and regional blood flow in areas of chronic hypoperfusion, suggesting that EPCs contribute to the homeostasis and repair of the cerebral circulation and maintenance of brain metabolism.14 The reduction in the number of EPCs appears to result from a decreased production and an enhanced degeneration of EPCs during the atherosclerotic process. Moreover, risk factors for atherosclerosis might directly influence the mobilization and survival of EPCs by impairing the bioavailability of nitric oxide. These findings support the notions that EPCs play an important part in the pathogenesis of atherosclerotic disease and that the measurement of EPCs could improve risk stratification.
EPCs are mobilized from BM during acute ischemia and contribute to the neovascularization of ischemic tissues. Acute myocardial infarction is associated with mobilization and a rapid increase in circulating EPCs.28 In the same line of acute cardiovascular events, vascular trauma such as coronary bypass grafting or burn injury induces a rapid but transient mobilization of VEGFR2+/AC133+ EPCs.29 Clinical trials assessing the therapeutic potential of BM-derived mononuclear cells (a rich source of immature cells including EPCs) in hind-limb40,41 and cardiac42 ischemia have yielded promising results. BM-derived immature cells have also been shown to participate in neovascularization of ischemic brain after experimentally induced stroke.43 Immature cells, including CD34+ cells, have been shown to contribute to vasculature maintenance, not only as a pool of EPCs but also as the source of growth/angiogenesis factors. Stroke involves a complicated cascade of events involving cerebral ischemia; altered blood flow; disruption, inflammation, neuronal necrosis, and apoptosis of the blood-brain barrier; and neurological dysfunction.44-46 Which of these mechanisms are involved in the mobilization of EPCs is not clear, but vascular trauma and tissue ischemia appear to facilitate EPC mobilization to the peripheral pool, in part by the release of cytokines and vascular endothelial growth factor.
In initial observation studies, Taguchi et al.14 measured CD34+ cells by flow cytometry in 25 patients with an ischemic stroke. They found that the values peaked after 7 days and returned to baseline after 30 days. Ghani et al.15 reported that the number of clusters of rapidly adhering cells was decreased after stroke and in "stable CVD" compared to in controls free of vascular disease. They found that being older and the presence of CVD are generally independently related to a lower number of EPCs, with no significant increase in EPC numbers being observed in the weeks after acute or stable stroke. These conflicting results are probably due to a lack of corroborated methods that can precisely identify EPCs.
We recently analyzed the characteristics of EPCs in acute stroke patients, focusing on their differences according to the pathogenetic mechanism.20 We found that the number of CFU was lower in acute stroke patients than in control subjects, and much lower in patients with large-artery atherosclerosis than in those with cardioembolism. Our data are consistent with a previous report15 that EPC numbers differed significantly among acute stroke, stable stroke, and control subjects, and extend those observations by suggesting that CFU analysis can improve the understanding of stroke pathophysiology. We also demonstrated a relationship between the known surrogate markers for chronic vasculopathy, with the HbA1c level being an independent predictor for fewer CFU in a multivariate analysis. A previous study found that the number of EPCs was significantly related to the HbA1c and blood sugar levels in diabetics, and that improving glycemic control can significantly increase the number of EPCs.47 Our data showing that the degree of glycemic dysregulation affects EPC function are in line with this previous report. On the other hand, the outgrowing cells appeared in most of the stroke patients but only rarely in control subjects. It is possible that immediate endothelial or neural damage induced by stroke induces a compensatory BM overproduction of progenitor cells for damage repair. It should also be emphasized that indexes for neurological damage, such as the NIHSS (National Institutes of Health Stroke Scale) score and infarct volume, were associated with the number of outgrowth cells. Our results indicated that the numbers of CFU and outgrowing cells represent markers of the accumulated vascular risk and response to ongoing tissue damage, respectively.
But do higher EPC numbers during the acute stage reverse the consequences of ischemia and improve prognosis? In observational studies involving patients with myocardial infarction, higher numbers of EPCs indeed relate to a better prognosis, more myocardial salvage,48 viability, and perfusion,49 and more collaterals in the ischemic zone.50 Human and animal model studies have shown that EPCs play a major role in angiogenesis and regeneration of ischemic brain tissue. However, the impact of the number of circulating EPCs on clinical outcomes after stroke remains uncertain. A recent clinical study found that the number of circulating EPCs was significantly higher in patients with acute ischemic stroke than in at-risk control subjects, and that the magnitude of this difference is directly related to the functional outcome.18 One prospective study involving 48 stroke patients showed that the EPC increase during the first week was independently associated with a good outcome at 3 months.17 This favorable effect on the primary variable was supported by the reduction of infarct growth and neurological improvement at days 7 and 90. These findings are in line with experimental and human studies suggesting that EPCs mediate endothelial cell regeneration and neovascularization, and that EPCs participate in the cerebral neovascularization processes present in the adult brain after ischemia. Further prospective investigations of patients with CVD to establish a prognostic value of EPCs in ischemic stroke would be very useful.
Mature nervous tissue has been considered incapable of cell renewal and structural remodeling for a long time, especially in mammals. However, stem cells are likely to replenish cells that are lost by physiological turnover, as well as in pathological conditions. It has recently been shown that appropriate in vitro and in vivo stimuli can induce adult somatic stem cells obtained from BM and PB to differentiate into neural-like cells.51-53 There have been intensive efforts over the past decade to develop therapeutic strategies for promoting revascularization of ischemic tissues. Since arteriogenesis and angiogenesis can be triggered by ischemia as native compensatory mechanisms,54 the discovery that EPCs are present in human PB raises the possibility of EPCs-mediated therapeutic neovascularization as a novel option for the treatment of ischemic diseases. The isolation and expansion of EPCs might be specifically useful for identifying therapeutic approaches targeting the progression and recurrence of stroke. Asahara et al.3 found transplanted EPCs in the endothelium of newly formed vessels in previously ischemic animal limbs. In other animal experiments, EPC administration also resulted in increased blood flow in ischemic zones and a decrease in limb loss.6 In experimentally induced cardiac ischemia, administration of progenitor cells resulted in neovascularization and a smaller infarcted area, although the involved mechanisms remained unclear.55
Brain repair after stroke is a formidable task, because the lesions are often large and involve cells of all types, resulting in the loss of many complex synaptic connections. EPC transplantation has been used to minimize the effects of ischemic stroke in animal models.56-58 Taguchi et al.56 demonstrated that the systemic administration of human CD34-positive cells accelerated neovascularization in the cerebral ischemic zone 48 h after stroke in a mouse model. This treatment also increased neurogenesis and improved functional indexes in these animals. Similarly, human cord blood administered intravenously to rats after stroke was able to enter the brain and dispersed in the ischemic brain microenvironment, resulting in improved functional recovery compared to controls.57 BM-derived cells have also been shown to participate in neovascularization processes in the adult brain of mice following ischemia.58 Neurological functions after chronic cerebral ischemia were considerably better in rats intracerebrally transplanted with PB stem cells than in vehicle-treated control rats. PB stem cells transplanted into rats were observed to migrate toward infarcted cerebral zones and to differentiate into neurons, glial cells, and endothelial cells, thus enhancing neuroplasticity in the ischemic brain.59 The intravenous injection of PB stem cells into rats early after focal cerebral ischemia reduced lesion volumes and improved regional cerebral blood flow and cognitive functions.60 The use of cells derived from PB or BM, including EPCs, provides two main advantages over the use of other cell types: (1) it avoids ethical limitations because there is no need to work with fetal or embryonic tissue, and (2) there is a host of experience on the use of hematopoietic progenitor cells in hemato-oncology research, and therefore there is considerable knowledge of treatment tolerability and side effects. Furthermore, neovascularization and neurogenesis might be tightly linked in the brain. A rich vascular environment, along with the generation of other nurturing neuronal mediators by EPCs (e.g., VEGF, FGF2, and IGF-1) enhances subsequent neuronal progenitor migration to the damaged area, followed by their maturation and survival when EPCs have stimulated the formation of increased vascular channels.
We have previously described the in vitro expansion and characterization of two types of progenitor cells from the PB of acute stroke patients.19 These cells are referred to as NOCs, in accordance with their morphological characteristics and protein or gene expression profiles. If neural progenitors could be isolated from human PB in sufficient quantities to permit harvesting, theoretically they would offer unlimited therapeutic possibilities. NOCs express immature neuronal markers over at least five passages, while maintaining rates of expansion characteristic of progenitor cells. Furthermore, the expressions of these markers are maintained in subclones obtained from these cells, and these subclones can be induced to undergo uniform differentiation into neurons in a chemically defined medium. Since PB-derived neural progenitor cells can be isolated without risk or potential side effects, their storage, expansion, and differentiation abilities makes them valuable candidates for transplantation therapy. Moreover, we found that intracerebral grafts of purified unmodified neural progenitor cells survived, migrated, and differentiated into neuronal phenotypes in ischemic rat brains.19 The exploitation of adult stem cells with neuronal characteristics-preferably obtained from easily accessible tissues-represents the best way of approaching cell therapy in stroke patients.
In future studies on the role of EPCs in stroke, several caveats should be borne in mind. First, we consider that the greatest current challenge in the field of EPC research is the lack of appropriate cell-surface markers specific for the identification of EPCs. Second, the laboratory technique of EPC quantification should be standardized as much as possible, and the timing of blood sampling (directly after the stroke or in the stable phase) should be considered, since the values obtained could differ between the acute and chronic phases. Third, the role of EPCs in the pathophysiology of CVD is no more than speculative at present. Therefore, it would be wise to further investigate the role of EPCs in different forms of stroke before planning clinical trials involving EPC injections. We also believe that the remarkable healing potential already demonstrated by EPC transplantation in experimentally induced stroke will become a clinical reality. However, we consider that clinical trials at this stage are premature and could be counterproductive. We suggest that this issue would be best progressed by standardizing certain aspects of basic research, especially the outcome measures, to facilitate comparisons between studies. In addition, long-term studies are required to determine whether cell-enhanced recovery is sustained, and to elucidate the tumorigenic potentials of cells. Furthermore, we must learn how to influence the pathological tissue environment and how this is related to repair. Other critical challenges include ensuring adequate characterization, manufacture, and quality control of cells, standardizing cell preparation protocols, and developing more definitive markers for stem cells. Identification of cell-surface markers for true stem cells would allow stem cell populations to be enriched or purified directly from heterogeneous cells.
EPCs or stem cells isolated from elderly stroke patients might retain dysfunctional characteristics, and therefore have reduced abilities to augment therapeutic regeneration. The expansion of cells in culture is an attractive strategy because it induces the potentiation of dysfunctional stem cells. Aside from these issues concerning ex vivo manipulation, considerable time is required to culture sufficient adult stem cells ex vivo to meet therapeutic requirements, and thus there is a need for an off-the-shelf supply of adult stem cells in the form of a cell bank that would allow physicians and surgeons to use stem cells directly at the point of care, rather than limiting their use to elective procedures.
EPCs hold great promise in CVD research, both as a marker for an increased risk of stroke and as a therapeutic agent after stroke. EPCs could change pathophysiological and therapeutic concepts, which will hopefully improve clinical treatments in vascular neurology. Recent work has provided a better understanding of these cells compared to what was known a decade ago. However, despite the promising results from various investigations (including our own), larger prospective studies should investigate this possible causation in order to confirm their role and applicability. Future studies should focus on establishing definitive markers of EPCs, better isolation methods, and the in-depth mechanisms underlying their beneficial effects.
Figures and Tables
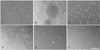
Fig. 1
Serial images of human peripheral-blood mononuclear cells (PBMNCs) cultured in endothelial growth media (EGM) to produce various types of progenitor cells. Phase-contrast images show the maturation of PBMNCs obtained from stroke patients during the culture immediately (A) and 7 days (B and C) after plating. Long-term cultures produced a heterogeneous population of cells (D) and led to a cobblestone (E) or palisading (E) outgrowth of cells. Scale bars: 30 µm (C), 60 µm (A and B), and 150 µm (D-F).
Acknowledgments
This research was supported by the Stem Cell Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, Republic of Korea (Grant No. SC4120).
References
1. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997. 349:1498–1504.

2. Hurtado O, Pradillo JM, Alonso-Escolano D, Lorenzo P, Sobrino T, Castillo J, et al. Neurorepair versus neuroprotection in stroke. Cerebrovasc Dis. 2006. 21:Suppl 2. 54–63.

3. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997. 275:964–967.

4. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003. 348:593–600.

5. Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999. 18:3964–3972.

6. Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000. 105:1527–1536.

7. Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999. 5:434–438.

8. Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001. 89:E1–E7.

9. Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest. 2000. 106:571–578.

10. Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002. 106:2781–2786.

11. Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005. 45:1449–1457.

12. Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005. 111:2981–2987.

13. Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005. 353:999–1007.

14. Taguchi A, Matsuyama T, Moriwaki H, Hayashi T, Hayashida K, Nagatsuka K, et al. Circulating CD34-positive cells provide an index of cerebrovascular function. Circulation. 2004. 109:2972–2975.

15. Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, et al. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005. 36:151–153.

16. Lau KK, Chan YH, Yiu KH, Li SW, Tam S, Lau CP, et al. Burden of carotid atherosclerosis in patients with stroke: relationships with circulating endothelial progenitor cells and hypertension. J Hum Hypertens. 2007. 21:445–451.

17. Sobrino T, Hurtado O, Moro MA, Rodríguez-Yáñez M, Castellanos M, Brea D, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007. 38:2759–2764.

18. Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, et al. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008. 39:69–74.

19. Jung KH, Chu K, Lee ST, Song EC, Sinn DI, Kim JM, et al. Identification of neuronal outgrowth cells from peripheral blood of stroke patients. Ann Neurol. 2008. 63:312–322.

20. Chu K, Jung KH, Lee ST, Park HK, Sinn DI, Kim JM, et al. Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke. 2008. 39:1441–1447.

21. Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999. 284:1168–1170.

22. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.

23. Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000. 290:1775–1779.

24. Keller G. Marshak DR, Gardner RL, Gottlieb D, editors. The hemangioblast. Stem Cell Biology. 2001. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press.
25. Rafii S. Circulating endothelial precursors: mystery, reality, and promise. J Clin Invest. 2000. 105:17–19.

26. Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004. 110:3300–3305.

27. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999. 85:221–228.

28. Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001. 103:2776–2779.

29. Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001. 88:167–174.

30. Khan SS, Solomon MA, McCoy JP Jr. Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry B Clin Cytom. 2005. 64:1–8.

31. Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997. 90:5002–5012.

32. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000. 95:952–958.

33. Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005. 106:1525–1531.

34. George J, Shmilovich H, Deutsch V, Miller H, Keren G, Roth A. Comparative analysis of methods for assessment of circulating endothelial progenitor cells. Tissue Eng. 2006. 12:331–335.

35. Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004. 24:288–293.

36. Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000. 105:71–77.

37. Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002. 105:546–549.

38. Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001. 104:191–196.

39. Fadini GP, Coracina A, Baesso I, Agostini C, Tiengo A, Avogaro A, et al. Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke. 2006. 37:2277–2282.

40. Taguchi A, Ohtani M, Soma T, Watanabe M, Kinosita N. Therapeutic angiogenesis by autologous bone-marrow transplantation in a general hospital setting. Eur J Vasc Endovasc Surg. 2003. 25:276–278.

41. Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002. 360:427–435.

42. Hamano K, Nishida M, Hirata K, Mikamo A, Li TS, Harada M, Miura T, et al. Local implantation of autologous bone marrow cells for therapeutic angiogenesis in patients with ischemic heart disease: clinical trial and preliminary results. Jpn Circ J. 2001. 65:845–847.

43. Beck H, Voswinckel R, Wagner S, Ziegelhoeffer T, Heil M, Helisch A, et al. Participation of bone marrow-derived cells in long-term repair processes after experimental stroke. J Cereb Blood Flow Metab. 2003. 23:709–717.

44. Yang GY, Pang L, Ge HL, Tan M, Ye W, Liu XH, et al. Attenuation of ischemia-induced mouse brain injury by SAG, a redox-inducible antioxidant protein. J Cereb Blood Flow Metab. 2001. 21:722–733.

45. Sairanen T, Carpén O, Karjalainen-Lindsberg ML, Paetau A, Turpeinen U, Kaste M, et al. Evolution of cerebral tumor necrosis factor-alpha production during human ischemic stroke. Stroke. 2001. 32:1750–1758.

46. Graham SH, Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2001. 21:99–109.

47. Kusuyama T, Omura T, Nishiya D, Enomoto S, Matsumoto R, Takeuchi K, et al. Effects of treatment for diabetes mellitus on circulating vascular progenitor cells. J Pharmacol Sci. 2006. 102:96–102.

48. Numaguchi Y, Sone T, Okumura K, Ishii M, Morita Y, Kubota R, et al. The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation. 2006. 114:1 Suppl. I114–I119.

49. Döbert N, Britten M, Assmus B, Berner U, Menzel C, Lehmann R, et al. Transplantation of progenitor cells after reperfused acute myocardial infarction: evaluation of perfusion and myocardial viability with FDG-PET and thallium SPECT. Eur J Nucl Med Mol Imaging. 2004. 31:1146–1151.

50. Lev EI, Kleiman NS, Birnbaum Y, Harris D, Korbling M, Estrov Z. Circulating endothelial progenitor cells and coronary collaterals in patients with non-ST segment elevation myocardial infarction. J Vasc Res. 2005. 42:408–414.

51. Goolsby J, Marty MC, Heletz D, Chiappelli J, Taskko G, Yarnell D, et al. Hematopoietic progenitors express neural genes. Proc Natl Acad Sci U S A. 2003. 100:14926–14931.

52. Sanchez-Ramos JR, Song S, Kamath SG, Zigova T, Willing A, Cardozo-Pelaez F, et al. Expression of neural markers in human umbilical cord blood. Exp Neurol. 2001. 171:109–115.

53. Mezey E, Key S, Vogelsang G, Szalayova I, Lange GD, Crain B. Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci U S A. 2003. 100:1364–1369.

55. Yoon YS, Lee N, Scadova H. Myocardial regeneration with bone-marrow-derived stem cells. Biol Cell. 2005. 97:253–263.

56. Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004. 114:330–338.

57. Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001. 32:2682–2688.

58. Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002. 90:284–288.






