Abstract
Background
Impairment of cognitive function is often present in patients with carotid artery stenosis but the details of this dysfunction have rarely been reported. Our purpose was to elucidate the cognitive dysfunction in patients with unilateral severe carotid stenosis using comprehensive neuropsychological testing, and also to identify the specific underlying clinical and radiological factors.
Methods
We analyzed the results of neuropsychological testing, the clinical history, and MR findings in 16 consecutive patients with angiographically proven severe (70-99%) stenosis of the extra cranial internal carotid artery (ICA). Cognitive functions were examined using the Seoul Neuropsychological Screening Battery and the Neglect Battery. We excluded patients with cortical infarction and those with contra lateral ICA occlusion or severe stenosis.
Results
Our comprehensive neuropsychological testing revealed obvious cognitive deficits in all patients with unilateral severe ICA stenosis, the most common being frontal executive impairment. The mean cognitive score on the memory test was also significantly lower in patients with symptomatic ICA stenosis than in asymptomatic patients (29.33±10.98, mean±SD, p < 0.05). The total score on the global cognitive test was significantly lower in patients with an ischemic lesion on MRI than in no lesion patients (113.23±34.78, p < 0.05). The presence of symptoms related to the ICA stenosis was related to cognitive dysfunction even when there were no ischemic lesions on MRI. SPECT revealed ipsilateral cortical hypoperfusion in 9 of 12 patients (75%).
Carotid stenosis is an important risk factor for stroke. The major cause of cerebral ischemia in patients with stenosis of the internal carotid artery (ICA) is intracranial arterial obstruction due to thromboembolism.1,3 recent studies have also demonstrated the importance of hemodynamic factors to predicting the risk of ischemic stroke after ICA stenosis. Impairment of cognitive function has often been reported in patients with carotid artery stenosis, especially those who experience a major stroke.4,15 However, few studies have employed comprehensive neuropsychological testing to provide detailed descriptions of the cognitive impairment patterns related to ICA stenosis in patients with minor stroke or even in asymptomatic patients. This study analyzed the detailed pattern of the reduced neuropsychological test performance with the aim of identifying the specific clinical and radiological factors that influence the effects of carotid stenosis on cognitive function.
The study cohort comprised 86 consecutive patients who were referred or admitted to the Department of Neurology, Eulji Medical Center, Daejeon, and whose intracranial or extra cranial arteries were evaluated using four-vessel angiography between February 2005 and January 2006. We enrolled 28 patients who had severe (70-99%) stenosis of the extra cranial ICA on angiography. Exclusion criteria were (1) combined intracranial severe stenosis or occlusion, (2) contra lateral ICA occlusion or severe stenosis, (3) significant disability (modified Rank in grade of ≥3), (4) large cortical infarct or basal ganglia, thalamus, or brainstem infarction, (5) recent stroke (less than 1 month after onset), (6) concomitant psychiatric and/or other neurological disease,(7) a geriatric depression score of ≥18, (8) major ischemic stroke, (9) subcortical small-vessel disease compatible with Binswanger's disease, (10) previous dementia, and (11) other medical disease that affected the performance in neuropsychological tests. These criteria resulted in the inclusion of 16 patients in the study.
A routine preprocedure stroke workup was performed in all cases using brain MRI and MRA. The MR studies were performed using a standard device with a 1.5-T magnet (GE Medical Systems, Milwaukee, WI; ver. 11.0 software platform). Standard clinical imaging sequences included axial T1-, T2-, and diffusion-weighted images, MRA at the circle of Willis, two-dimensional time-of-flight MRI of the cervical carotid arteries, and dynamic contrast-enhancedelliptic centric MRA of the aortic arch through the circle of Willis. Two neuroradiologists independently read all the MRI scans. The stenotic vessels were measured on conventional angiograms according to the method described by the North American Symptomatic Carotid Endarterectomy Trial (NASCET) for cervical carotid lesions (Fig. 1).6
Brain perfusion SPECT images were acquired in 12 patients (75%) using a triple-head gamma camera (Prism 3000; Picker International, Cleveland, OH) with a fan-beam collimator. Approximately 555 MBq of Tc-99m-HMPAO was administered.
Cognition was examined using the Seoul Neuropsychological Screening Battery and the Neglect Battery. All neuropsychological assessments were administered on the same day to each patient. The tests administered for the examined areas of neuropsychological functioning were as follows: attention and working memory (Digit Span Forward and Backward Test), frontal function tests (motor impersistence test, contrasting program, go/no-go test, alternating square and triangle test, Luria loop test, Stroop test, and word fluency tests), verbal memory test (Korean version of the Hopkins Verbal Learning Test), visuospatial function and visual memory test (Rey Osterrieteh complex figure tests), language tests (comprehension item, fluency item, repetition, reading, writing, and the Korean version of the Boston Naming Test), and Neglect Battery tests (line bisection, letter cancellation test, two-daisy drawing test, and modified Ogden test). Cognitive scores in each patient were compared with the standard scores of normal healthy subjects who were matched with respect toage, sex, and duration of education. The Korean version of the Mini-Mental State Examination (K-MMSE) was also applied.
Demographic and vascular factors were compared by χ2 tests, and the results from each neuropsychological test were assessed using the t-test. Separate analyses were performed for the following between-patients subgroups: right- vs. left-side stenosis, symptomatic vs. asymptomatic patients, and ischemic lesions on MRI vs. no ischemic lesions.
The characteristics of the study participants are listed in Table 1. The severe ICA stenosis was on the left and right in eight patients each. The mean age of the patients was 68.44 years (SD, 4.2 years range, 61-79 years). Eleven patients were male, and the mean duration of education was 6.69 years (SD, 3.4 years range, 2-16 years). The clinical manifestations were minor stroke and transient ischemic attack (TIA) in 10 and 3 patients, respectively, and 3 patients were asymptomatic. Risk factors and comorbidities included arterial hypertension (15 patients), diabetes mellitus (6 patients), tobacco use (9 patients), hyperlipidemia (4 patients), and coronary heart disease (4 patients). The demographics and vascular affective risk factors did not differ significantly between patients with right- and left-side ICA stenosis (p > 0.05).
Localized infarction in the periventricular or subcortical white matter (but not the cortex) on MRI was present in 11 of the 16 patients (69%). Six patients had an infarction in the left periventricular area, such as in Figure 1, and the others had similar lesions on the right side. There were no cases of a large hemispheric lesion that included the cortex. Seven of the patients (44%) had combined contralateral ICA mild stenosis, and four (25%) had mild ipsilateral intracranial artery stenosis (Table 1). Perfusion SPECT revealed ipsilateral frontotemporoparietal cortical hypoperfusion in seven patients, no perfusion defect in two patients, ipsilateral frontal cortical hypoperfusion in two patients, and ipsilateral fronto-parietal cortical hypoperfusion in one patient.
Neuropsychological assessments revealed that 14 of the 16 patients had cognitive deficits in more than one domain of attention, frontal executive function, visual and verbal memory impairments, or visuospatial dysfunction. Frontal executive impairment was found in all patients. Memory dysfunction was found in 13 of the patients (63%); of which 8 showed working memory impairment and 9 had verbal memory impairment. Six of the nine patients with verbal memory problems showed retrieval deficit, and the other three patients showed the pattern of an encoding deficit. Eight of the 13 patients had visual memory impairment, of which 6 patients showed the pattern of retrieval deficit and 2 showed the pattern of an encoding deficit. Four of the 13 patients with memory problems had both verbal and visual memory problems. Attention problems were found in 8 of the 16 patients (50%), and visuospatial dysfunction was present in 7 of the 16 patients (43%). The score in the memory domain of the neuropsychological test was significantly worse in patients with left-side ICA stenosis than in those with right-side ICA stenosis (p < 0.05) (Table 2). The mean cognitive score in the memory test was also significantly lower in patients with symptomatic ICA stenosis than in asymptomatic patients (p < 0.05) (Table 3). The frontal executive function score was significant worse in patients with an ischemic lesion on MRI than in those without such a lesion (p < 0.05) (Table 4).
Patients with carotid stenosis are at risk of cognitive loss and vascular dementia, and this may also be a modifiable risk factor for cognitive decline.7-15 There have been many reports of a relationship between ICA stenosis and cognitive function. A search of the literature cited on Medline and Psychic between 1980 and 1999 by Bakker et al. revealed that 14 of 18 studies concluded that cognitive deficits are present in patients with either symptomatic or asymptomatic carotid obstruction, with a mild and diffuse detrimental effect of carotid stenosis on cognitive function. But those authors identified methodological problems such as in the neuropsychological testing, and suggested the need for further prospective research to confirm their findings. The inclusion of patients with a past history of stroke or vertebrobasilar insufficiency in some studies reduces their validity, and most reports have not commented on the degree of stenosis. The lack of comparable control groups also makes the interpretation of neuropsychological data problematic.
Our detailed and comprehensive neuropsychological testing revealed obvious cognitive deficits in all patients, with the severity varying from very mild to severe. The most common cognitive dysfunction was mild-to moderate frontal executive impairment. These findings are consistent with the patterns of deficits observed in previous studies.7-9,13 A semantic or phonemic word fluency tests and the Stroop test are the most sensitive for assessing frontal executive function. The memory test scores were significantly worse in patients with symptomatic ICA stenosis and left-side stenosis than in those with asymptomatic ICA stenosis and right-side stenosis. Memory impairment mainly affects the working memory resulting in forgetfulness, and problems with retrieval deficit reflect frontal dysfunction, whereas encoding deficits reflect hippocampus dysfunction. Neglect, visuospatial dysfunction, and the performances of the verbal and visual memory did not differ significantly between the left and right sides. Vascular lesions on MRI were associated with significant differences in frontal executive function scores. We do not know the exact reason why the neuropsychological results did not reflect the functional cognitive asymmetry, but we presumed that these nonspecific neuropsychological differences between the right and left hemispheres were more attributable to diffuse cerebral dysfunction from hemodynamic factors, rather than to localize ischemic lesions.
Our study appears to show that neuropsychological tests are more sensitive than MRI to early changes in cerebral functioning due to atherosclerosis. We therefore recommend the routine inclusion of cognitive testing in future clinical trials designed to evaluate the cognitive function of patients with ICA stenosis, even those who are asymptomatic. The Tromso Study also showed that asymptomatic carotid stenosis in the absence of an MRI vascular lesion was associated with neuropsychological impairment.8 We ascertained that the K-MMSE was not sufficiently sensitive to detect modest improvements in cognitive performance in the patients with carotid stenosis, since our comprehensive neuropsychological tests revealed that even patients with K-MMSE scores of 26.28 points exhibited obvious cognitive dysfunction. However, Pettigrew et al. suggested that the MMSE would be useful in predicting mortality or the first occurrence of stroke, TIA, or myocardial infarction in patients with asymptomatic carotid artery stenosis.9
Carotid stenosis may be a marker of intracerebral or generalized atherosclerosis, perhaps resulting from microcirculatory disturbances due to microangiopathy and a reduction in cerebral perfusion due to impaired vasoreactivity with increased small-vessel resistance.1-3,18,19 The carotid lesions could affect the development of lacunar infarction in dependent areas of the perforating arteries via a homodynamic or micro embolic mechanism.1,3,16 The relationship between silent infarction and carotid disease has not been clarified, probably because of the difficulties of applying the appropriate methodology (e.g., patient selection, obtaining samples from outpatients, and defining comparative groups). The mechanism of cognitive impairment associated with carotid stenosis remains unclear, but possible mechanisms are lacunar infarction and small-vessel disease.17,18 A lacunar or ischemic lesion could arise from emboli producing multiple subclinical infarcts, or through small-vessel disease affecting subcortical structures and deep white matter. A lacunar infarction associated with ICA stenosis disturbs the prefrontal subcortical loops involved in executive control, but not global or memory function. The mechanisms underlying the relationship between lacunar infarction and executive dysfunction include disconnection or diaschisis of frontal-lobe function. Memory disturbance in patients with carotid stenosis may also be attributable to subcortical vascular disease causing frontal cortical dysfunction in those without hippocampus dysfunction caused by Alzheimer's disease. However, since our study excluded small-vessel disease, and even included patients without infarction on MRI, another mechanism may underlie the executive dysfunction. Bakker et al. found that cognitive function did not improve in patients with lactate present in noninfarcted white matter of the hemisphere ipsilateral to the ICA occlusion, and suggested that this reflects persisting hemodynamic impairment.19 We do not know the definitive reason for the differences in memory impairment between left- and right-side ICA stenosis and between symptomatic and asymptomatic patients with ICA stenosis. However, we consider that these differences were not due to a higher rate of MRI lesions such as lacunar or cortical infarcts or white-matter hyper intensities in our study, because all patients with leftside stenosis and symptomatic patients did not have ischemic lesions on MRI, and also most patients with memory impairment showed diffuse cortical hypoperfusion in SPECT. Six of the 16 patients received carotid stent intervention therapy, and after 3 months of stent placement 3 of the patients with diffuse cortical hypoperfusion in SPECT showed dramatic cognitive improvement in neuropsychological testing (data not shown). It is not clear whether interventions such as stent placement or endarterectomy would have resulted in cognitive improvement in most of the patients with ICA stenosis, since it has been reported that some patients show significant improvements, others are unchanged, and others show deterioration.20-26 The reason for these varying results are also unclear, but may depend on factors such as the assessed variable, the magnitude of micro emboli production, and the side of the affected vasculature.24 Although our sample was small, we suggest that the pattern and degree of cognitive impairment should be assessed along with clinical symptoms, stenosis side, SPECT findings, and the presence of vascular lesions on MRI in patients with severe ICA stenosis when determining the intervention therapy.
The limitations of our study are that it did not include a control group, functional imaging such as SPECT was not performed in all patients, and the sample was very small. The strengths of this study are the use of a standardized extensive neuropsychological test battery, and the use of MRI and four-vessel angiography for accurate detection of the vascular lesion and the degree of ICA stenosis. To our knowledge, this is the first investigation of the relationship between neuropsychological performance and clinical symptoms, the side of stenosis, and the presence of vascular lesions on MRI. We found that the presence of symptoms in ICA stenosis - even in the absence of a vascular lesion on MRI - has an important effect on cognitive function. Previous studies have also suggested the limitations of neuroimaging in assessing the presence of vascular dementia or vascular cognitive impairment.8,27 We therefore suggest that an additional mechanism beyond the structural lesion (e.g., chronic hypoperfusion) affects the cognition in patients with high-grade ICA stenosis. We further suggest that detailed comprehensive neuropsychological testing should be used to detect vascular cognitive impairment in patients with carotid stenosis, and that chronic hypoperfusion ischemia is the cause of vascular cognitive impairment
Figures and Tables
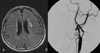
Figure 1
Images obtained in a 65-year-old man with a minor completed stroke. (A) FLAIR image showing small ischemic lesions in the left deep white-matter area. (B) Carotid angiogram showing severe stenosis of the proximal internal carotid artery as measured by the NASCET method.
References
1. Inzitari D, Eliasziw M, Sharpe BL, Fox AJ, Barnett HJ. Risk factors and outcome of patients with carotid artery stenosis presenting with lacunar stroke. North American Symptomatic Carotid Endarterectomy Trial Group. Neurology. 2000. 54:660–666.

2. Inzitari D, Eliasziw M, Gates P, Sharpe BL, Chan RK, Meldrum HE, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 2000. 342:1693–1700.

3. Eliasziw M, Kennedy J, Hill MD, Buchan AM, Barnett HJ. North American Symptomatic Carotid Endarterectomy Trial Group. Early risk of stroke after a transient ischemic attack in patients with internal carotid artery disease. CMAJ. 2004. 170:1105–1109.

4. Yamauchi H, Fukuyama H, Nagahama Y, Oyanagi C, Okazawa H, Ueno M, et al. Long-term changes of hemodynamics and metabolism after carotid artery occlusion. Neurology. 2000. 54:2095–2102.

5. Klijn CJM, Kappelle LJ, Tulleken CAF, van Gijn J. Symptomatic carotid artery occlusion. A reappraisal of hemodynamic factors. Stroke. 1997. 28:2084–2093.
6. Fisher M, Martin A, Cosgrove M, Norris JW. The NASCETACAS plaque project. North American Symptomatic Carotid Endarterectomy Trial. Asymptomatic Carotid Atherosclerosis Study. Stroke. 1993. 24:I24–I25.
7. Bakker FC, Klijn CJM, Jennekens-Schinkel A, Kappelle LJ. Cognitive disorders in patients with occlusive disease of the carotid artery: a systemic review of the literature. J Neurol. 2000. 247:669–676.

8. Mathiesen EB, Waterloo K, Joakimsen O, Bakke SJ, Jacobsen EA, Bonaa KH. Reduced neuropsychological test performance in asymptomatic carotid stenosis:The Tromso Study. Neurology. 2004. 62:695–701.

9. Pettigrew LC, Thomas N, Howard VJ, Veltkamp R, Toole JF. Low mini-mental status predicts mortality in asymptomatic carotid arterial stenosis. Asymptomatic Carotid Atherosclerosis Study investigators. Neurology. 2000. 55:30–34.
10. Bakker FC, Klijn CJM, van der Grond J, Kappelle LJ, Jennekens-Schinkel AJ. Cognition and quality of life in patients with carotid artery occlusion: a follow-up study. Neurology. 2004. 62:2230–2235.

11. Rao R. The role of carotid stenosis in vascular cognitive impairment. Eur Neurol. 2001. 46:63–69.

12. Hamster W, Diener HC. Neuropsychological changes associated with stenoses or occlusions of the carotid arteries. A comparative psychometric study. Eur Arch Psychiatry Neurol Sci. 1984. 234:69–73.

13. Naugle RI, Bridger's SL, Delaney RC. Neuropsychological signs of asymptomatic carotid stenosis. Arch Clin Neuropsychol. 1986. 1:25–30.

14. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991. 325:445–453.
15. Van den Burg W, Saan RJ, Van Zomeren AH, Boontje JH, Haaxma R, Wichmann TE. Carotid endarterectomy: does it improve cognitive or motor functioning? Psycho Med. 1985. 15:341–346.

16. Tejada J, Diez-Tejedor E, Hernandez-Echebarria L, Balboa O. Does a relationship exist between carotid stenosis and lacunar infarction? Stroke. 2003. 34:1404–1409.

17. Reed BR, Eberling JL, Mungas D, Weiner M, Kramer JH, Jagust WJ. Effects of white matter lesions and lacunar on cortical function. Arch Neurol. 2004. 61:1545–1550.

18. Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey DJ, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004. 63:246–253.

19. Bakker FC, Klijn CJM, Jennekins-Schinkel A, van der Tweel I, van der Grond J, van Huffelen AC, et al. Cognitive impairment is related to cerebral lactate in patients with carotid artery occlusion and ipsilateral transient ischemic attacks. Stroke. 2003. 34:1419–1424.

20. King GD, Gideon DA, Haynes CD, Dempsey RL, Jenkins CW. Intellectual and personality changes associated with carotid endarterectomy. J Clin Psychol. 1977. 33:215–220.

21. Hemmingsen R, Mejsholm B, Vorstrup S, Lester J, Engell HC, Boysen G. Carotid surgery, cognitive function, and cerebral blood flow in patients with transient ischemic attacks. Ann Neurol. 1986. 20:13–19.

22. Kelly MP, Garron DC, Javid H. Carotid artery disease, carotid endarterectomy, and behavior. Arch Neurol. 1980. 37:743–748.

23. Irvine CD, Gardner FV, Davies AH, Lamont PM. Cognitive testing in patients undergoing carotid endarterectomy. Eur J Vasc Endovasc Surg. 1998. 15:195–204.

24. Lunn S, Crawley F, Harrison MJ, Brown MM, Newman SP. Impact of carotid endarterectomy upon cognitive functioning. A systematic review of the literature. Cerebrovasc Dis. 1999. 9:74–81.

25. Heyer EJ, Adams DC, Solomon RA, Todd GJ, Quest DO, McMahon DJ, et al. Neuropsychometric changes in patients after carotid endarterectomy. Stroke. 1998. 29:1110–1115.

26. Ballard CG, Burton EJ, Barber R, Stephens S, Kenny RA, Kalaria RN, et al. NINDS AIREN neuroimaging criteria do not distinguish stroke patients with and without dementia. Neurology. 2004. 63:983–988.

27. Pohjasvaara T, Erkinjuntti T, Vataja R, Kaste M. Dementia three months after stroke Baseline frequency and effect of different definitions of dementia in the Helsinki Stroke Aging Memory Study (SAM) cohort. Stroke. 1997. 28:785–792.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download