Hypertensive encephalopathy (HE) is characterized by acute onset, severe arterial hypertension, confusion, headache, and seizure. HE is usually reversible, but failure to treat hypertension promptly may produce a fatal outcome.1-3 The typical abnormality on MRI is signals of increased intensity in the white matter of both occipital regions consistent with edema. The term "reversible posterior leukoencephalopathy syndrome" has been used to describe the characteristic neuroimaging features in HE.4 Extension of the edema into the brainstem, basal ganglia, and cerebellum is reportedly rare, and is usually associated with cortical lesions.5 Involvement of the brainstem in addition to the supratentorial lesions has been rarely reported, and is termed hypertensive brainstem encephalopathy (HBE).6-8 We report two cases of HBE without accompanying occipital lobe changes.
CASE REPORT
Case 1.
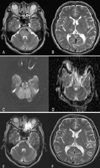
A 53-year-old man complained of paresthesia in both hands and headache. His past medical history was unremarkable, and a neurological examination was normal. His initial blood pressure was 170/100 mm Hg, and fundoscopy showed hypertensive retinopathy. Areas of increased signal were present in the pons, right thalamus, and cerebellum in brain MRI fluid-attenuated inversion recovery (FLAIR) and T2-weighted images, without any accompanying occipital lobe changes (Fig. 1-A, B). Diffusion-weighted imaging (DWI) was normal, but the apparent diffusion coefficient (ADC) map contained a high-intensity signal corresponding to that on T2-weighted images (Fig. 1-C, D). There was no contrast enhancement. A laboratory evaluation was unremarkable except for a creatinine level of 12.8 mg/dl. The results of a cerebrospinal fluid examination and nerve conduction study were normal. He underwent hemodialysis, and his blood pressure was normalized over the next several days, which resolved the paresthesia and headache. The normal neurological examination led to a preliminary diagnosis of HE, which was subsequently confirmed by the complete resolution of the previous MRI lesions after 2 weeks (Fig. 1-E, F).
Case 2.
A 45-year-old woman presented with left-sided weakness. Her past medical history was significant for poorly controlled hypertension and chronic renal insufficiency. Her blood pressure at admission was 190/130 mm Hg, and a neurological examination showed mild left hemiparesis. Her mental status and cranial nerve examination were normal. A laboratory evaluation was unremarkable except for a creatinine level of 3.2 mg/dl. DWI revealed a high-intensity signal in the right corona radiata. In addition, increased signals were noted in the pons, midbrain, and periventricular white matter in FLAIR and T2-weighted images without accompanying cortical lesions. Normal DWI and a high-intensity signal in the ADC map were evident in the area of the high-intensity signal on the T2-weighted image. However, despite the presence of extensive neuroimaging brainstem lesions, there were no symptoms or signs of brainstem dysfunction. Repeated MRI after the blood pressure was controlled revealed that the brainstem and periventricular white-matter lesions had resolved. She was discharged with mild left hemiparesis.
DISCUSSION
The clinical presentations of our patients were not consistent with HE, although they initially presented with severe hypertension, and subsequently improved after normalization of blood pressure. The MRI features of our patients were unusual in that they spared the parieto-occipital lobes and predominantly involved the brainstem. Although previous studies of HE have found brainstem abnormalities on MRI, such changes are usually associated with hemispheric abnormalities in the occipital regions.9,10 The posterior predilection is thought to be related to dense sympathetic innervation of the anterior circulation that protects neural structures if the blood pressure exceeds the limits of autoregulation.8
There are a few reports that the presence of brainstem or cerebellar edema on neuroimaging is the principal feature of HE. Our two patients had high-intensity signals in the brainstem on T2-weighted and FLAIR images. However, the signal intensity in DWI was similar to that in surrounding regions, whereas that of ADC mapping was higher, respectively. HE presenting with predominant involvement of the brainstem must be differentiated from brainstem infarction, brainstem tumor, encephalitis, and central pontine myelinolysis. Brainstem infarction was ruled out by the lack of major brainstem signs, normal DWI, and the rapid clinical recovery. Also, the absence of abnormal serum sodium levels, the clinical recovery, and the resolution of the MRI lesions reduced the possibility of central pontine myelinolysis being present.
It has been suggested that DWI and ADC values help to differentiate abnormal signals on T2-weighted and FLAIR images from brainstem infarction.6,11 The lack of an increased signal on DWI and the presence of increased ADC values suggest the absence of cytotoxic edema in regions of hyperintense T2-weighted lesions. Such cases would favor a diagnosis of HBE. According to the results of DWI and ADC mapping performed on our patients, most of the regions showing high-intensity signals on T2-weighted images demonstrated vasogenic edema rather than cytotoxic edema. These MRI changes may support the hypothesis that presence of severe hypertension exceeding the autoregulation limit results in segmental vasodilatation and increased vascular permeability, leading to vasogenic edema.5,12 Therefore, with proper application, DWI and ADC mapping can be conveniently utilized to differentiate HBE from other brain diseases, which show decreased ADC values consistent with cytotoxic edema.
The clinical manifestations of the brainstem variant are similar to those of the more common form of HE. One-third of patients reportedly have only hypertension as the precipitating factor, with most having comorbidities such as renal failure.8 Interestingly, despite the presence of extensive neuroimaging lesions, that study found few symptoms or signs of brainstem or cerebellar dysfunction, indicating clinical radiologic dissociation. Our patients did not have any clinical signs of brainstem dysfunction, as in the case described by Morello et al.13 We suggest that a typical characteristic of HBE is the paucity of clinical signs, which certainly bear no relationship to the severity indicated in MRI.
We have presented two patients with clinical and radiological features characteristic of HBE. Brainstem and cerebellar variants of HE are rare, and generally affect younger people with secondary hypertension such as renal failure. This diagnosis must be considered in the presence of severe hypertension with clinical radiologic dissociation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download