Abstract
Reiter's syndrome belongs to the family of spondyloarthropathies that usually present with a triad of arthritis, urethritis, and uveitis. The diagnostic criteria include clinical, radiological, and genetic findings, and the response to treatment. Nervous system involvement in Reiter's syndrome is extremely rare. We report here on a 36-year-old man who initially presented with progressive cervical myelopathy and was diagnosed as Reiter's syndrome 2 years later. The myelopathy was stable after treatment with methotrexate and sulfasalazine. This case suggests that Reiter's syndrome can present as progressive myelopathy and should be considered in the differential diagnosis of treatable myelopathies.
Reiter's syndrome is an aseptic arthritis that is triggered by an infectious agent located outside the joint. It has a strong association with human leukocyte antigen-B27 (HLA-B27). Enteric infection with salmonella or other microorganisms has been implicated as a trigger for this syndrome. Since Reiter's original description of the tetrad of urethritis, conjunctivitis, arthritis, and other mucocutaneous lesions in 1916, cases of multiple-organ involvement in patients with classic Reiter's syndrome have been documented.1 Central or peripheral nervous system involvement in Reiter's syndrome has been well recognized, but this particular condition is very uncommon. This is the first Korean report of the long-term clinical course of a patient with progressive myelopathy and Reiter's syndrome.
A previously healthy 36-year-old man presented with slowly progressive spastic paraparesis, impairment of vibration, and pain perception below the upper trunk associated with constipation, urinary difficulty, and sexual dysfunction. He had experienced lower abdominal pain and vomiting 7 months before the onset of these symptoms, and subsequently noticed a tingling sensation in the right leg and voiding difficulties.
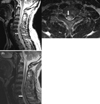
The neurologic examination performed at the time of his first visit to our clinic was compatible with a spinal cord lesion: there was bilateral hypesthesia below the T10 level, hyperactive knee and ankle jerks, and extensor plantar responses. Also, he experienced voiding difficulties and a urinary tract infection. T2-weighted magnetic resonance imaging (MRI) of the spine showed a lesion with a high signal intensity and mild swelling that was not enhanced after the administration of contrast material in the cervical spinal cord (Fig. 1). The cerebrospinal fluid (CSF) was clear and colorless with 5 white blood cells/µL, and the cells were mostly lymphocytes. Electrophoresis of CSF was negative for the oligoclonal band, and the IgG index was within the normal range. The laboratory findings for rheumatoid factor, antinuclear antibody, antineutrophil cytoplasmic antibodies, cryoglobulinemia, hepatitis B and C, and human immunodeficiency virus were negative. The erythrocyte sedimentation rate and C-reactive protein and complement levels (C3, C4, and CH50) were normal. The results for the visual evoked potential and brain MRI were negative. The patient was treated with steroid pulse therapy under a diagnosis of transverse myelitis. He complained of intermittent chest discomfort when subsequently visiting our clinic, but electrocardiography, cardiac enzymes, and echocardiography findings were normal.
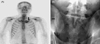
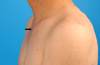
The first steroid pulse therapy provided temporary relief from bilateral hypesthesia and paresthesia, but he still experienced spastic paraparesis with painful tonic spasm and bladder dysfunction including recurrent urinary tract infection and nonspecific anterior chest tenderness, especially in the costochondral joint area. However, the spastic paraparesis slowly deteriorated and paresthesia relapsed in the lower extremities. Another two cycles of steroid pulse therapy were administered during the 2 years following the first treatment due to progression of sensory and motor symptoms. Follow-up spine MRI after the second steroid pulse therapy revealed recurrence of intramedullary myelopathy (Fig. 1-B). Two years after the onset of myelopathy, he developed painful swelling of his costochondral joints and both knees. A 99mTc methylene diphosphonate bone scan showed markedly increased uptake in the left costomanubrial junction and the first rib (Fig. 2-A). Pelvis radiographs showed ankylosis of bilateral hip joints and poorly delineated bilateral sacroiliac joints (Fig. 2-B). He subsequently displayed multiple pustular skin eruptions on the palms of the hands, soles of the feet, elbows, trunk (Fig. 3), and glans penis. He was positive for HLA-B27 antigen. Administration of methotrexate and sulfasalazine markedly reduced the patient's arthralgia and neurologic symptoms, which were stabilized by maintenance therapy with methotrexate and prednisolone.
The spondyloarthropathies comprise a diverse group of inflammatory arthritis conditions that share certain genetic predisposing factors and clinical features. The pathogenesis of spondyloarthropathies is still not well defined. Recent studies have provided insight into distinct pathogenetic mechanisms underlying ankylosing spondylitis and reactive arthritis that arise from a complex interplay between genetic factors (including HLA-B27) and environmental factors.2 The pathogenesis of Reiter's syndrome may involve molecular mimicry between bacterial fragments in synovial fluid and the HLA-B27 molecule. Most (70-80%) patients with Reiter's syndrome are positive for HLA-B27, as compared with only 6% of the general population. The arthritis may be perpetuated by the induction of cytotoxic T lymphocytes by microbial fragments in the joints, but these cytotoxic T lymphocytes have specificity for HLA-B27-positive cells. The presence of HLA-B27 may allow stronger or persistent microbial invasion.3
Reactive arthritis usually has a self-limited course of 3 to 12 months, but up to 50% of patients experience recurrent bouts of arthritis, and 15% to 30% of them develop chronic symptoms of the disease.4 Extra-articular manifestations such as ocular inflammation, enteritis, mucocutaneous lesions, urethritis, and (rarely) carditis provide essential support for a diagnosis of reactive arthritis. However, neurological complications are rare.5 There have been only a few case reports of polyneuropathy, cranial nerve palsy, or myelopathy in Reiter's syndrome.6,7
Whilst there were gastrointestinal symptoms in this case, stool examinations provided no laboratory evidence of preceding infection. There also was no preceding urethral discharge or notable infections. The patient visited our clinic at 7 months after the onset of the first symptoms. The high dose of steroids administered might have inhibited a systemic inflammatory reaction. According to a previous report, approximately only 60% of such cases have evidence of previous infection detected either by serology or by cultures from urogenital or stool samples.8 Nonsteroid anti-inflammatory drugs (NSAIDs) and sulfasalazine are effective treatments for reactive arthritis, and methotrexate can also be beneficial.9 Our patient did not respond to NSAIDs, but methotrexate and sulfasalazine relieved his neurological symptoms and arthralgia, making them stable during a 5-year follow-up.
The association between reactive arthritis and cervical myelopathy was not clear in this patient, but there were some features suggesting Reiter's syndrome as a cause of the myelitis. First, the gastrointestinal symptoms that appeared before the development of cervical myelopathy might have reflected a preceding infection that initiated an autoimmune reaction, which led to myelitis and systemic inflammation. Initial urinary tract infection and costochondral tenderness at the time of the first attack of myelitis might be indicative of Reiter's syndrome. Second, despite the application of steroid pulse therapy, recurrences of progressive myelopathy associated with systemic symptoms of the skin and joints, and the positivity for HLA-B27 suggest other causes of myelitis. Third, both neurological symptoms and the arthritis that was resistant to the steroid pulse therapy did not recur after sulfasalazine and methotrexate treatment.
In conclusion, Reiter's syndrome should be considered in the differential diagnosis of cases of progressive myelopathy with multiple arthritis, urethritis, and skin lesions.
Figures and Tables
Figure 1
(A) T2-weighted sagittal and axial spine MRI demonstrated an area of high signal intensity in the spinal cord at the level of C7 and Th1, and mild swelling of the cord (arrow). The lesion exhibited a slightly low signal intensity on precontrast T1-weighted imaging and no enhancement after contrast administration (arrow in right image). (B) Follow-up T2-weighted sagittal spine MRI after the first steroid pulse therapy revealed a new linear lesion with a high signal intensity in the posterior portion of the spinal cord at the level of C7 to Th1 (arrow).
References
1. Montanaro A, Bennett RM. Myelopathy in Reiter's disease. J Rheumatol. 1984. 11:540–541.
2. Kim TH, Uhm WS, Inman RD. Pathogenesis of ankylosing spondylitis and reactive arthritis. Curr Opin Rheumatol. 2005. 17:400–405.

3. Hermann E, Yu DT, Meyer zum Buschenfelde KH, Fleischer B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993. 342:646–650.

4. Colmegna I, Espinoza LR. Recent advances in reactive arthritis. Curr Rheumatol Rep. 2005. 7:201–207.

6. Unverferth DV, Beman FM, Ryan JM, Whisler RL. Reiter's aortitis with pericardial fluid, heart block and neurologic manifestations. J Rheumatol. 1979. 6:232–236.
7. Cuchacovich M, Gatica H, Contreras L. Neurologic involvement in Reiter's disease. Report of 2 cases. Rev Med Chil. 1991. 119:687–690.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download