Abstract
Progressive multifocal leukoencephalopathy (PML) is a rare disease that occurs mainly in immunocompromised patients. Despite the progressive nature of the disease, the changes on MRI during the disease course - which may help in monitoring the disease process - have seldom been reported. Here we describe a patient with polymerase-chain-reaction-proven PML examined using serial diffusion-weighted imaging (DWI) and apparent-diffusion-coefficient mapping. Magnetic resonance spectroscopy (MRS) revealed that the demyelinating process was more active without significant neuronal loss at the newer and advancing edge of a lesion than in the older central part of the lesion. This case shows that MRI findings such as DWI and MRS may improve the diagnosis and the understanding of the pathophysiology of PML.
Progressive multifocal leukoencephalopathy (PML) is a rare disease that occurs mainly in immunocompromised patients. Despite the progressive nature of the disease, the changes on serial diffusion-weighted imaging (DWI) and magnetic resonance spectroscopy (MRS) during the disease course - which may help in monitoring the disease process - have seldom been reported.
Here we demonstrate serial DWI and MRS findings of various lesion stages in a patient with polymerase chain reaction (PCR)-proven PML.
A 42-year-old previously healthy man presented with a 4-day history of nonfluent aphasia. His tendon reflexes, power, and coordination were normal. Routine laboratory investigations, including examination of the cerebrospinal fluid, produced normal findings. Brain computed tomography showed multiple low-density cerebral lesions. Magnetic resonance imaging (MRI) was performed, including DWI and apparent diffusion coefficient (ADC) mapping. Multiple lesions in the right parietal and left frontal white matter gave very high signals on T2-weighted images and low signals on T1-weighted images. DWI showed multiple high signals on bilateral subcortical areas (Fig. 1-A). With a provisional diagnosis of multiple cortical infarcts, an extensive etiological work-up was performed, but this was negative. The patient was transferred to the Department of Neurology and followed at an outpatient clinic.
Thirty days later the patient developed global aphasia and dysphasia, at which time MRI revealed marked progression of the cerebral lesion on both sides (Fig. 1-B). DWI showed extension of the previous lesions with diverse signals, notably with high signals on their medial borders. The ADC maps showed different regions within the lesions: the diffusion was normal to low at the medial border (0.83±0.04 mm3/s, mean±SD) but high at the center (1.92±0.08 mm3/s). Proton MRS was performed over the new and old lesions, and also over the normal brain parenchyma in healthy volunteers as a control (Fig. 2) since asymptomatic AIDS patients may have abnormalities in MRS.1 Choline (Cho), creatine (Cr), N-acetylaspartate (NAA), lactate, and lipids were evaluated in each spectrum, and NAA/Cr, NAA/Cho, and Cho/Cr ratios were calculated.
Cho/Cr, lipids/Cr, and lactate/Cr ratios were higher and the NAA/Cr ratio was lower in both PML lesions than in normal control. However, the pattern differed between new and old lesions, with the intensity on DWI being higher, the increase in Cho being greater, and the decrease in NAA being smaller at the newer and advancing edge of a lesion than in the older central part of the lesion, where the ADC value was high (Table 1).
The diagnosis of PML was subsequently established with positivity for serum anti-HIV antibody and PCR-based detection of JC virus DNA in the cerebrospinal fluid. The CD4 cell count was 10 cells/ml (normal range, 500-1000 cells/ml), and the HIV RNA level was 300,000 IU/ml. Highly active antiretroviral therapy (HAART) was initiated, and 2 months later the patient progressed to a bedridden state.
PML occurs mainly in immunocompromised patients, manifesting as a progressive decline in neurologic functions. However, PML should also be considered in the differential diagnosis of apparently healthy patients with focal deficits. The present case with PML was apparently healthy and presented with manifestations suggestive of a stroke, and showed a high signal intensity on DWI. However, the patient presented clinical features consistent with PML during the follow-up, including rapidly progressive neurological decline, suggesting nonvascular disease.
DWI may be useful for evaluating or monitoring disease activity and for the differential diagnosis of patients with atypical clinical features. The DWI findings of patients with PML differ in asynchronous lesions and are dependent on the stage, as in our case.2-4 Newer lesions and the advancing edges of large lesions had normal-to-low ADCs and gave a high signal on DWI, whereas the older lesions and the centers of the large lesions had increased ADCs and gave a low signal on DWI. These striking findings might help to differentiate PML from mimicking diseases, such as ischemic stroke, multiple sclerosis, or MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes), especially in young and apparently healthy patients such as the case reported here.
MRS has been used for histopathologic correlations in brain tumors, multiple sclerosis, epilepsy, and neurodegenerative disorders. There are reported data of homogeneous spectroscopic patterns in PML characterized by a decrease in the NAA/Cr ratio and an increase in the lactate/Cr, Cho/Cr, and lipids/Cr ratios relative tohealthy control subjects or on the contralateral side5-7; however, most of these investigations did not measure asynchronous lesions. In our patient, the MRS pattern differed depending on the stage, with the Cho increase being greater and the NAA decrease being smaller in newer lesions than in older ones, suggesting the presence of an ongoing demyelinating process without severe neuronal or axonal loss. These MRS findings are in good agreement with previous pathologic findings showing ongoing demyelination with swollen oligodendrocytes at the border of a lesion, but sparse oligodendrocytes, large extracellular space, and axonal disruption in the center of the lesion.8
There is increasing awareness about PML because the use of many immunosuppressant treatments in patients with multiple sclerosis and organ transplants may predispose them to JC virus reactivation, leading to PML.9,10
This emphasizes the need for prompt diagnosis and monitoring of the disease process. Although we could not perform serial MRS studies during the course of HAART, MRS has the advantage of determining the effects of such an active treatment modality, as well as for evaluating the clinical course of patients with PML, which cannot be obtained in a postmortem investigation. Future studies should attempt to confirm these observations in more patients with PML.
Figures and Tables
Figure 1
ADC maps and DWI at the first and second examinations. (A) Axial DWI showed a high signal intensity at the left frontal and right parietal lesions. (B) Follow-up DWI performed 1 month after the first MRI showed extension of the previous lesion, with a high signal intensity on the medial border. ADC value was low to normal at the medial border of the lesion, whereas it was high in the center of the lesion.
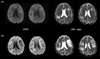
Figure 2
Proton-MRS spectra acquired from white matter in newer (A) and older (B) PML lesions and in a control subject (C). Cho, lactate, and lipids were higher and NAA was lower in PML lesions than in normal white matter. Resonance values: NAA; 2.01 ppm, Cr; 3.04 ppm, Cho; 3.22 ppm, lactate; 1.33 ppm, and lipids; 0.90 ppm.

References
1. Wilkinson ID, Miller RF, Miszkiel KA, Paley MN, Hall-Craggs MA, Baldeweg T, et al. Cerebral proton magnetic resonance spectroscopy in asymptomatic HIV infection. AIDS. 1997. 11:289–295.

2. Henderson RD, Smith MG, Mowat P, Read SJ. Progressive multifocal leukoencephalopathy. Neurology. 2002. 58:1825.

3. Ohta K, Obara K, Sakauchi M, Obara K, Takane H, Yogo Y. Lesion extension detected by diffusion-weighted magnetic resonance imaging in progressive multifocal leukoencephalopathy. J Neurol. 2001. 248:809–811.

4. Mader I, Herrlinger U, Klose U, Schmidt F, Kuker W. Progressive multifocal leukoencephalopathy: analysis of lesion development with diffusion-weighted MRI. Neuroradiology. 2003. 45:717–721.

5. Chang L, Ernst T, Tornatore C, Aronow H, Melchor R, Walot I, et al. Metabolite abnormalities in progressive multifocal leukoencephalopathy by proton magnetic resonance spectroscopy. Neurology. 1997. 48:836–845.

6. Iranzo A, Moreno A, Pujol J, Marti-Fabregas J, Domingo P, Molet J, et al. Proton magnetic resonance spectroscopy pattern of progressive multifocal leukoencephalopathy in AIDS. J Neurol Neurosurg Psychiatry. 1999. 66:520–523.

7. Simone IL, Federico F, Tortorella C, Andreula CF, Zimatore GB, Giannini P, et al. Localised 1H-MR spectroscopy for metabolic characterisation of diffuse and focal brain lesions in patients infected with HIV. J Neurol Neurosurg Psychiatry. 1998. 64:516–523.

8. Bergui M, Bradac GB, Oguz KK, Boghi A, Geda C, Gatti G, et al. Progressive multifocal leukoencephalopathy: diffusion-weighted imaging and pathological correlations. Neuroradiology. 2004. 46:22–25.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download