Abstract
Poststroke hypotension may be related to early neurological deterioration and infarct progression by decrease of cerebral perfusion, particularly in patients with ischemic penumbra. However, optimal management guideline of blood pressure in the setting of acute stroke is absent, and remains a matter of debate. We report here a patient who had early neurological deterioration accompanied by systemic hypotension. Phenylephrine-induced hypertensive therapy by imaging-based decision making successfully restored neurologic dysfunction in this patient.
A spontaneous increase in blood pressure (BP) is common in patients with acute ischemic stroke. The cause of this elevation has been attributed to autonomic effects of stroke, stress of hospitalization, and the teleological need for the brain and ischemic penumbra to increase cerebral blood flow. Blood pressure tends to decline in the first few days to weeks after stroke onset, even without pharmacological intervention.
The importance of BP management after stroke for secondary prevention has been well established. Unlike chronic stroke, however, BP reduction in acute stroke phase may lead to different and untoward effects. Vemmos et al. confirmed that poor outcome was seen in patients with either very high or very low initial BP, consistent with a 'U-shaped' curve.1 Therefore, the optimal management of BP in the setting of acute stroke is uncertain, and remains a matter of debate and demands further investigation.
The early perfusion- (PWI) and diffusion-weighted magnetic resonance imaging (DWI) have been proposed as a useful method to evaluate ischemic penumbra. In patients with perfusion-diffusion mismatch, poststroke hypotension may cause early neurological deterioration and infarct progression by decrease of cerebral perfusion. The recanalization of occluded vessels using thrombolytic therapy or interventional procedures has been usually considered. Alternatively, restoration of neurologic dysfunction may be accomplished by way of BP elevation ('induced hypertension') to increase cerebral blood flow to threatened areas, particularly in whom thrombolytic or interventional therapy is not indicated. Here, we describe a patient with perfusion-diffusion mismatch who had early neurologic deterioration accompanied by systemic hypotension. The neurological deterioration was completely resolved following induced hypertensive therapy.
A 68-year-old man suddenly developed mild aphasia, dysarthria and right arm weakness. These symptoms occurred after excessive sweating during hiking in the mountains on day time. The patient arrived at emergency room 16 hours 30 minutes after symptom detection. The symptoms had an improving course at that time. He had hypertension and chronic renal failure, and had been treated with medications during the past 20 years. In addition, he had coronary heart disease and underwent coronary artery bypass graft surgery 1 year ago.
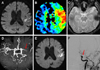
On the initial neurological examination, he was alert and had mild motor-dominant aphasia. Mild dysarthria without facial palsy was noted. Motor and sensory function tests were normal. Deep tendon reflexes and cerebellar function tests were also normal. Initial National Institutes of Health Stroke Scale (NIHSS) score was 4. The result of his chest x-ray and electrocardiogram showed normal configuration. Initial BP was 128/73 mmHg. A brain magnetic resonance imaging (MRI) scan taken 17 hours after the onset, DWI showed acute infarct in the left middle cerebral artery (MCA) territory with a large perfusion defect. A brain magnetic resonance angiogram (MRA) showed severe stenosis or occlusion in left proximal MCA (Fig. 1). Transthoracic echocardiogram revealed normal cardiac chamber size, normal ejection fraction (66%) and mid-posterior wall thinning with akinesia in the left ventricle. Antiplatelet agents with aspirin and cilostazol and volume replacement with normal saline were administered.
At hospital day 4, neurological deterioration occurred to him. He had drowsy mental status, severe global aphasia, gaze preference to the left, right facial palsy, and right side hemiparesis (Medical Research Council grade IV). NIHSS score was 12. Blood pressure was 87/52 mmHg at that time. The cause of BP drop was not clear. The blood tests including cardiac enzyme (creatinine kinase MB fraction, myoglobin, troponin-I) and electrocardiogram were unremarkable, but chest x-ray revealed mild pulmonary congestion. Despite severe clinical deterioration, the follow-up DWI showed some new scattered lesions in the left MCA territory, but did not show significant lesion growth compared with initial DWI. These findings suggested that the mechanism responsible for the neurological deterioration might be prolonged hemodynamic compromise rather than artery-to-artery embolism. Then catheter angiography was performed to consider intra-arterial recanalization therapy such as thrombolysis or stenting. 100,000 unit of urokinase was applied intra-arterially without any effect. Final angiogram showed that left M1 was still occluded. Then, BP elevation was tried by administration of normal saline 100 mL/hr and volume expander, hydroxyethyl starch 40 mL/hr for the increase of collateral circulation. Follow-up BP was elevated to 100/55 mmHg, and then 120/70 mmHg 4 hours after neurological deterioration. However, his neurological deficits persisted throughout the treatment with intensive volume expander. Then, phenylephrine (60 µg/min) was administered. His systolic (SBP) and diastolic blood pressure (DBP) elevated more than 150 and 80 mmHg. While the systemic BP increased rapidly 1 hour after the administration of phenylephrine, the neurological deficits improved slowly, though steadily, over the following 24 hours. His neurological symptoms were fully recovered 36 hours later. We tapered out the pressor drugs, because his BP was maintained without further medication. The figure 2 shows the temporal relationship between BP and neurological symptoms. He had no adverse events related to pressor drugs. Antihypertensive medication was administered to the patient 7 days later, with his SBP being maintained between 140 and 150 mmHg. He has not developed recurrent neurological symptoms for the following 1 year period.
Poststroke hypotension is not rare phenomenon. According to previous reports, it could have been associated with neurological deterioration and poor outcome. In a retrospectively analysis of 17,398 patients in the International Stroke Trial (IST),2 18% of patients had SBP <140 mmHg, early (2-week) death increased by 17.9% for every 10 mmHg SBP below 150 mmHg.3 As potential causes of poststroke hypotension, previous studies4-6 suggested the poor left ventricular function and low cardiac output from heart disease (e.g., congestive heart failure, coronary heart disease, arrhythmia), the use of aggressive antihypertensive medication, dehydration (e.g., diarrhea, poor oral intake), and septic condition due to hidden infection. In our patient, the chest x-ray showed mild pulmonary congestion at the time of neurological deterioration. Consequently, this finding supported that patient's hypotensive episode may be associated with pre-existing heart disease.
In patients with perfusion-diffusion mismatch, poststroke hypotension may cause early neurological deterioration and infarct progression by decrease of collateral blood flow.7 Besides coronary heart disease, our patient had perfusion-diffusion mismatch accompanied with intracranial large artery disease, which suggest that this patient was particularly vulnerable to hemodynamic compromise and had a high risk of early neurological deterioration. Despite clinical deterioration, follow-up DWI did not show additional significant infarcts. Therefore, we presumed that the main mechanism of neurological deterioration in this patient was hypoperfusion.
Although pressor-induced hypertensive therapy is a standard treatment for cerebral ischemia in patients with vasospasm after subarachnoid hemorrhage, there are few experimental or human data to support this practice following acute ischemic stroke. In the ischemic penumbra as an area of brain surrounding of infarcted tissues, reversible electrical failrue was present, but irreversible cell death had not yet occurred. Increasing systemic BP in patients with low systemic BP could rescue the ischemic penumbra from cerebral infarct by increasing intraluminal hydrostatic pressure, opening collateral channels and improving cerebral blood flow and perfusion to penumbral ischemic tissues.8
We used phenylephrine as a pressor therapy. Phenylephrine (a selective α1-agonist) increases BP by peripheral vasoconstriction, without substantial direct cerebral vasoconstriction, due to a low density of α1-receptors in cerebral vessels.9 It is less likely to cause of tachyarrhythmias, having negligible β-agonist actionand increasing myocardial oxygen demand compared to other pressor agents. Hillis et al10 found significant improvement of NIHSS, cognitive score and volume of hypoperfused tissue on PWI, in the phenylephrine-treated group with no adverse events such as cardiac ischemia or hemorrhagic conversion of infarct. We could not demonstrate elevated cerebral perfusion followed by induced hypertensive therapy in this case, because follow-up PWI was not performed.
Current guidelines about the management of poststroke hypotension provide no objective clarification as to the appropriate management, which is a reflection of the paucity of evidence in this field. It is difficult to select patients who could benefit from pressor therapy and to define an absolute BP level at which pressor therapy should be considered. The most suitable candidate and therapeutic BP window for benefit is uncertain. In this patient, the elevation of systemic BP after the intra-arterial thrombolytic therapy might increase the risk of hemorrhage. However, we considered that benefit of reperfusion by induced hypertensive therapy might be higher than the risk of hemorrhage, because our patient received relatively small dose of urokinase (100,000 units) intra-arterially. Moreover, we tried to maintain the systolic BP no more than 160 mmHg. Large, well-designed trials are needed to clarify the management of arterial hypotension after acute stroke. Currently, a trial testing the utility of phenylephrine as a pressor therapy in the setting of stroke (Controlling Hypertension and Hypotension Immediately Post-Stroke [CHHIPS]) is ongoing.11 Before strong evidence is available, patients with significant SBP fall (e.g., > 20 mmHg) and neurological deterioration, particularly accompanied with pre-existing large artery disease or cardiac dysfunction, may be considered as a therapeutic target. We regard that our patient fit well into this category. The reason for the lag between BP elevation induced by phenylephrine and subsequent neurologic improvement is not clear. Although SBP was elevated to more than 150 mmHg after phenylephrine infusion, the BP might be still below optimal range. Another possible explanation is that the time lag exists from BP elevation to increase of collateral circulation and cerebral perfusion and ultimately to clinical improvement. Stunned brain syndrome, which can sometimes be observed after reperfusion therapy,12 is less likely, because the BP after phenylephrine infusion was not higher than BP before neurological deterioration.
Although we report a single case, this report reinforces the utility of DWI and PWI in identifying the mechanism of early neurological deterioration and in guiding the clinical decision for administration of the most appropriate management. Active induced hypertensive therapy can be considered, when a viable ischemic penumbra exists on early MRI and a neurological deterioration is accompanied by hemodynamic compromise.
Figures and Tables
Figure 1
A 68-year-old man presented with mild aphasia, dysarthria and right arm weakness (NIHSS score = 4). Initial MRI shows acute ischemic lesions in the left middle cerebral artery (MCA) territory on diffusion weighted image (DWI) (A) with a larger perfusion defect on mean transit time map (B) not accompanied with clot or hemorrhage on gradient echo image (GRE) (C) and severe stenosis or occlusion of left proximal MCA on MR angiography (D). At hospital day 4, global aphasia, left gaze preference, right facial palsy, and right hemiparesis grade IV developed (NIHSS score = 12). DWI shows some additional scattered ischemic lesions in the left MCA territory (E). Intra-arterial thrombolysis was performed, and urokinase of 100,000 unit was applied. Final angiogram shows that left MCA is still occluded (F).
References
1. Vemmos KN, Tsivgoulis G, Spengos K, Zakopoulos N, Synetos A, Manios E, et al. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J Intern Med. 2004. 255:257–265.

2. The International Stroke Trial (IST). A randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International stroke trial collaborative group. Lancet. 1997. 349:1569–1581.
3. Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA. Blood pressure and clinical outcomes in the international stroke trial. Stroke. 2002. 33:1315–1320.

4. Rigaud AS, Seux ML, Staessen JA, Birkenhager WH, Forette F. Cerebral complications of hypertension. J Hum Hypertens. 2000. 14:605–616.

5. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heartoutcomes prevention evaluation study investigators. N Engl J Med. 2000. 342:145–153.

6. Vemmos KN, Spengos K, Tsivgoulis G, Zakopoulos N, Manios E, Kotsis V, et al. Factors influencing acute blood pressure values in stroke subtypes. J Hum Hypertens. 2004. 18:253–259.

7. Serena J, Rodriguez-Yanez M, Castellanos M. Deterioration in acute ischemic stroke as the target for neuroprotection. Cerebrovasc Dis. 2006. 21:Suppl 2. 80–88.

8. Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981. 12:723–725.

9. Bevan JA, Duckworth J, Laher I, Oriowo MA, McPherson GA, Bevan RD. Sympathetic control of cerebral arteries: Specialization in receptor type, reserve, affinity, and distribution. Faseb J. 1987. 1:193–198.

10. Hillis AE, Ulatowski JA, Barker PB, Torbey M, Ziai W, Beauchamp NJ, et al. A pilot randomized trial of induced blood pressure elevation: Effects on function and focal perfusion in acute and subacute stroke. Cerebrovasc Dis. 2003. 16:236–246.

11. Potter J, Robinson T, Ford G, James M, Jenkins D, Mistri A, et al. CHHIPS (Controlling Hypertension and Hypotension Immediately Post-Stroke) pilot trial: Rationale and design. J Hypertens. 2005. 23:649–655.

12. Alexandrov AV, Hall CE, Labiche LA, Wojner AW, Grotta JC. Ischemic stunning of the brain: Early recanalization without immediate clinical improvement in acute ischemic stroke. Stroke. 2004. 35:449–452.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download