Abstract
Background
Reduced cerebral blood flow and microvascular abnormalities have been suggested as the vascular pathogenesis of Alzheimer's disease (AD). Transcranial Doppler sonography (TCD) can be used as a noninvasive method for measuring cerebral vasomotor reactivity (VMR) which represent the capability of arterioles to dilate and constrict in order to maintain cerebral blood flow.
Objective
The objective of this study was to determine whether VMR is decreased in AD patients. Methods: Seventeen consecutive patients who met NINDS-ADRDA criteria for AD, and 17 age- and sex-matched controls were included in this study. MRI and MRA were performed for the grading of white-matter lesions. Patients with cerebral infarct or stenosis of the middle cerebral artery (MCA) were excluded. The fixed TCD probe was used to monitor the mean flow velocity (MFV) in the MCA. A 6-L rebreathing bag was applied to patients for at least 5 minutes to elevate the CO2 concentration, which was continuously monitored with a capnometer. VMR was calculated as the percentage change in the MFV.
Results
Baseline characteristics - including cerebrovascular risk factors, grades of white-matter lesions, baseline MFV, and pulsatility index - did not differ between the two groups. Mini-Mental State Examination score was significantly low in AD group (20.5 vs. 27.5, p<0.05). VMR was significantly reduced in AD group both in the right-side (24.5% vs. 36.6%, p<0.05) and left-side (20.7% vs. 34.1%, p<0.05) MCAs.
Several epidemiological1-4 and neuroimaging5-7 studies performed during the last 10have provided evidence supporting the vascular pathogenesis of Alzheimer's disease (AD). These studies have suggested that vascular risk factors directly reduce cerebral perfusion to a critical level of dysfunction, enhancing neuronal death in AD.8,9 A recent population-based study using transcranial Doppler sonography (TCD) strongly supports this hypothesis by demonstrating that cerebral hypoperfusion precedes and possibly contributes to the onset of clinical dementia.10 Another study has suggested that cerebral vasomotor reactivity (VMR) is a significant predictor of cognitive decline in AD patients.11
VMR refers to the capability to dilate or constrict cerebral arterioles in response to metabolic stimuli, such as CO2 or acetazolamide, which may be decreased in large-vessel occlusive disease or small-vessel microangiopathy.12 However, VMR has rarely been studied in AD patients, and it has not been clarified whether VMR is lower in AD patients than in nondemented subjects. Using a TCD rebreathing method, we investigated differences in VMR between AD patients and control subjects, which may be related to the vascular pathogenesis of AD.
Dementia patients aged from 55 to 80 years who met the NINDS-ADRDA criteria for AD were included in this study.13 Age- and sex-matched control subjects without dementia were chosen from those who had visited the neurology clinic or health promotion center in Seoul National University Boramae Hospital. The Mini-Mental State Examination (MMSE), MRI, and MRA were applied to all subjects. Subjects who showed territorial infarcts, multiple lacunes, stenosis of the middle cerebral artery (MCA), or bilateral suboptimal temporal window were excluded. Because VMR can be affected by certain drugs such as statins14 and angiotensin-converting enzyme inhibitors,15 those using such drugs were also excluded. All subjects gave their informed consent.
Changes in white-matter lesion severity in MRI were quantified used the Rotterdam Scan Study (RSS) scale,16 which separately scores the periventricular regions (periventricular score range: 0 to 9) and the volume of subcortical white-matter lesions (subcortical score range: 0 to 29.5 mL).
With the subject in a supine position, the TCD probe was used in a fixed position to monitor the mean flow velocity (MFV) in the MCA. A rebreathing method was adopted for elevating the CO2 concentration. A 6-L rebreathing bag with several pores was applied to patients via a facial mask for at least 5 minutes, and the CO2 concentration was continuously monitored with a capnometer. Subjects with inadequate CO2 retention (≤ 45 mmHg) were excluded from the analysis. Baseline blood pressure, bilateral MFV, and pulsatility index (PI) were obtained, and MFV was continuously monitored and digitally recorded for later off-line analysis. VMR was calculated as the percentage change in the MFV according to VMR = (MFVhypercapnia-MFVnormocapnia)100/MFVnormocapnia). Validation of this method and normal reference values have been presented previously.17
Student's t-test was used for intergroup comparisons of continuous values, and the chi-square test was used for comparisons of categorical variables. The correlations of VMR with the MMSE score and the grade of white-matter lesions were analyzed by bivariate correlation analysis. A probability value of p<0.05 was considered significant. All data are presented as mean±SD values.
Seventeen AD patients and 17 control subjects were recruited (age 67.1±5.9 years, range 55 to 78 years; 7 males and 10 females per group). The MMSE score was significantly lower in AD patients than the control subjects (22.1±4.9 vs. 28.7±0.6, p=0.042). Other baseline characteristics - including cerebrovascular risk factors, medications, and grades of white-matter lesions - did not differ significantly between the two groups, as summarized in Table 1.
Four and one AD patients showed suboptimal temporal windows on the right and left sides, respectively. Therefore, we also excluded the corresponding TCD values of the matched control subjects from the analysis. The baseline MFV and PI also did not differ between the AD patients and the control subjects. However, VMR was significantly decreased in AD patients in both the right- and left-side MCAs (Fig. 1): 57.7±22.2 and 36.8±26.0 in control subjects and AD patients, respectively, on the right side (p=0.037); and 58.7±21.1 and 37.2±22.7 on the left side (p=0.014). VMR was linearly correlated on the right and left sides (Pearson's correlation coefficient=0.883, p=0.001).
VMR and MMSE scores of AD patients were not significantly correlated either in the right-side (Pearson's correlation coefficient=0.264, p=0.324) or left-side (Pearson's correlation coefficient=0.095, p=0.758) MCA (Fig. 2). VMR of AD patients was not correlated with either the volume of subcortical white-matter lesions or the grade of periventricular white-matter lesions (all p>0.05).
Whether AD is a pure neurodegenerative disorder or has contributions from vascular disease remains controversial. In the present study, VMR was lower in patients with mild-to-moderate AD than in age- and sex-matched control subjects. Several TCD studies have shown that the blood flow velocity is decreased in AD,18,19 and one study found an association between VMR and future cognitive decline in AD.11 However, the present report is the first to compare VMR between AD patients and nondemented subjects.
In the RSS study, VMR was reduced in subjects with cognitive decline but not in patients with clinical dementia.10 In addition, the cerebral blood MFV was correlated with both the cognitive decline and the clinical dementia severity.10 However, that study involved only 13 AD patients among 1730 subjects, which might have resulted in a lack of statistical power, and it was suggested that reduced VMR in AD was related to significant vascular comorbidity.10 It has recently been reported that reduced cerebrovascular reactivity predicts cognitive decline in AD patients.11 However, that report presented no information on the level of VMR in the AD patients, and hence on whether VMR decreased with the disease progression.11
In the present study, VMR but not the blood flow velocity was lower in AD patients than in the controls. This suggests that the pathologic changes of AD are more closely related to VMR than to the flow velocity itself. It is known that a reduced VMR is correlated with the severity of microangiopathy, which increases vascular resistance.20,21 In addition, reduced VMR is representative of a reduced vasomotor reserve, which is prominent in hemodynamically significant stenosis or occlusion of the internal carotid artery.10,20 Because cerebrovascular CO2 reactivity is influenced by atherosclerosis through disturbance of the integrity and function of the arterial wall, a reduction in VMR to hypercapnia is indicative of cerebral small-vessel pathology.10,20
Nevertheless, whether reduced VMR is correlated with decreased cerebral perfusion is not yet certain. Critical threshold cerebral hypoperfusion may result in changes to the capillaries, including basement membrane thickening, endothelial cell compression, degeneration of pericytes, and lumen distortion.9 These changes can reduce the capacity of the arterioles to dilate in response to CO2 stimuli, which represents VMR. Consequently, reduced VMR is likely to be related to (or at least associated with) critical hypoperfusion. Direct measurement of cerebral perfusion and metabolism using SPECT or PET will provide more information about the significance of a reduced VMR.
It remains unclear whether the decreased perfusion reflects the diminished demand caused by advanced neurodegeneration, or whether cerebral hypoperfusion precedes and contributes to dementia and neurodegeneration. The decreased delivery of oxygen due to the decreased cerebrovascular reserve can lead to hypoxia in vulnerable areas of the brain, such as watershed areas and the hippocampus,10 and can negatively affect cognitive function. In addition, any events that reduce cerebral blood flow and perfusion can trigger cerebral injury in patients with a reduced VMR.
We found no correlation between the MMSE score and VMR in the present study. In addition, VMR was not correlated with the grade of white-matter lesions. However, these results are not conclusive given the small number of patients in our study. Therefore, future studies should include a larger population of AD patients, and also evaluate cognitive function in more detail (i.e., using instruments additional to the MMSE). In addition, follow-up measurements of VMR and assessments of their correlation with the degree of cognitive decline are warranted.
Several methods have been used to measure VMR. The semiclosed rebreathing method as used in this study is easy to apply to and well tolerated by elderly demented patients, who may be uncooperative. The value of VMR measured by this method is comparable to that obtained by CO2-inhalation and breath-holding techniques.17 In the present study, an adequate CO2 retention of near 50 mmHg or more was obtained in all subjects.
In conclusion, we have shown that VMR is reduced in patients with AD, which may be indicative of the microangiopathy of AD. Screening of cerebrovascular reserve by measuring VMR using TCD could represent a much-needed, reliable, safe, and cost-effective technique for patients at risk of dementia. Future studies should check the validity of these experimental and hypothesis-generating pilot results.
Figures and Tables
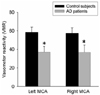 | Figure 1Difference in VMR values. VMR was significantly reduced in AD patients in the MCAs on both sides, being 57.7±22.2 and 36.8±26.0 in control subjects and AD patients, respectively, on the right side, and 58.7±21.1 and 37.2±22.7 on the left side (*p<0.05 vs. control). |
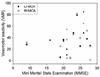 | Figure 2Correlation between MMSE score and VMR. Scatterplot shows no correlation between MMSE score and VMR both in the right-side (p=0.758) and left-side (p=0.324) MCAs. |
Table 1
Baseline characteristics and VMR

While baseline MFV values did not differ between the two groups, VMR was lower in the AD group than in the control group. Statistical significances (p values) were tested by Student's t-test for continuous values, and by the chi-square test for categorical variables.
ACE; angiotensin-converting enzyme, DBP; diastolic blood pressure, SBP; systolic blood pressure
References
1. Breteler MMB. Vascular risk factors for Alzheimer's disease: an epidemiologic perspective. Neurobiol Aging. 2000. 21:153–160.
2. Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002. 1:61–77.

3. Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997. 349:151–154.

4. Guo Z, Qiu C, Viitanen M, Fastbom J, Winblad B, Fratiglioni L. Blood pressure and dementia in persons 75+ years old: 3-year follow-up results from the Kungsholmen Project. J Alzheimers Dis. 2001. 3:585–591.

5. Johnson KA, Albert MS. Perfusion abnormalities in prodromal AD. Neurobiol Aging. 2000. 21:289–292.

6. Kogure D, Matsuda H, Ohnishi T, Asada T, Uno M, Kunihiro T, et al. Longitudinal evaluation of early Alzheimer's disease using brain perfusion SPECT. J Nucl Med. 2000. 41:1155–1162.
7. Rodriguez G, Vitali P, Calvini P, Bordoni C, Girtler N, Taddei G, et al. Hippocampal perfusion in mild Alzheimer's disease. Psychiatry Res. 2000. 100:65–74.

8. de la Torre JC. Is Alzheimer's disease preceded by neurodegeneration or cerebral hypoperfusion? Ann Neurol. 2005. 57:783–784.

9. de la Torre JC. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004. 3:184–190.

10. Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005. 57:789–794.

11. Silvestrini M, Pasqualetti P, Baruffaldi R, Bartolini M, Handouk Y, Matteis M, et al. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke. 2006. 37:1010–1015.

12. Hetzel A, Braune S, Guschlbauer B, Dohms K. CO2 reactivity testing without blood pressure monitoring? Stroke. 1999. 30:398–401.

13. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984. 34:939–944.

14. Sander K, Hof U, Poppert H, Conrad B, Sander D. Improved cerebral vasoreactivity after statin administration in healthy adults. J Neuroimaging. 2005. 15:266–270.

15. Walters M, Muir S, Shah I, Lees K. Effect of perindopril on cerebral vasomotor reactivity in patients with lacunar infarction. Stroke. 2004. 35:1899–1902.

16. de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001. 70:9–14.

17. Heo JH, Lee YS. Measurement of cerebral vasomotor reactivity with semi-closed rebreathing method: validation study and normal reference values. J Neurol Sci. 2005. 238:supp 1. A414. Abstract.
18. Ries F, Horn R, Hillekamp J, Honisch C, Konig M, Solymosi L. Differentiation of multi-infarct and Alzheimer dementia by intracranial hemodynamic parameters. Stroke. 1993. 24:228–235.

19. Reed BR, Jagust WJ, Seab JP, Ober BA. Memory and regional cerebral blood flow in mildly symptomatic Alzheimer's disease. Neurology. 1989. 39:1537–1539.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download