Abstract
We report a patient with multiple simultaneous embolic infarctions with localized hemorrhagic conversion. A 75-year-old male patient had several silent microbleeds (SMBs) exclusively in the cerebral cortex, and underwent angioplasty and stenting for bilateral carotid stenosis. He subsequently experienced embolic infarctions in the cortex and the striatum: the cortical infarction, where an SMB had been present, showed hemorrhagic conversion, whereas the striatal infarction did not. This case suggests that SMBs are indicators of an underlying hemorrhage-prone state.
Silent microbleeds (SMBs) visualized by gradientecho (GRE) magnetic resonance imaging (MRI) may have a close regional association with intracerebral hemorrhages1 and may be a risk factor for hemorrhagic transformation or subsequent hemorrhages in the patients with ischemic stroke.2 However, there have been contradictory data regarding the association: a recent retrospective analysis suggested that the presence of a small number of SMBs is not related to the risk of thrombolysis-induced hemorrhage.3 Thus, the impact of SMBs on the subsequent symptomatic hemorrhage is not yet fully understood, especially in the case of the hemorrhagic conversion of an embolic infarction. In this context, we report an interesting case of multiple embolic infarctions: a hemorrhagic infarction in the cerebral cortex where there were several SMBs versus an ischemic infarction in the basal ganglia where SMBs were not present.
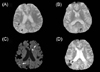
A 75-year-old-male with a history of a right middle cerebral artery infarction 5 years previously was admitted for the management of bilateral proximal internal carotid artery (ICA) stenoses diagnosed by magnetic resonance angiography. An acute infarct lesion was not observed, but GRE-sequence MRI revealed several SMBs exclusively in the bilateral cerebral cortex (Fig. 1-A and 1-B). SMBs were not found in the basal ganglia or thalamus. Because severe stenoses of bilateral proximal ICAs (>70%, NASCET criteria) were confirmed by cerebral angiography, balloon angioplasty and stenting was performed on the left proximal ICA immediately and on the right proximal ICA 1 month later. There were no initial complications after stenting, such as hyperperfusion syndrome or embolic infarction.
Twenty-five days after the last stenting, the patient became slightly obtunded and was unable to lift his left arm. Brain diffusion-weighted and GRE-sequence MRI was performed at 2 days after ictus. Two acute ischemic stroke lesions were identified in the striatum and the cortex by diffusion-weighted imaging (DWI) (Fig. 1-C). Interestingly, multiple embolic strokes had resulted in an embolus flowing to the right parietal lobe where an SMB had been present on the previous GRE-sequence MRI investigation, which caused a hemorrhagic infarction, whereas no hemorrhage was found around the striatal lesion (Fig. 1-D).
SMBs are usually caused by chronic hypertension,4 and an increased burden therefrom is also correlated with increased hypertension.5 The close relationship with chronic hypertension is also supported by pathological data indicating that SMBs are hemosiderin-containing macrophages that appear around advanced hypertensive microangiopathic lesions such as lipohyalinosis.6 Furthermore, these lesions are strongly associated with the presence of intracerebral hemorrhage in patients with hypertension, with their cerebral locations coinciding with the locations of the intracerebral hemorrhage.1,7 Because SMBs and silent lacunar infarctions exhibit different topographical distributions,8 SMBs may be considered indicative of bleeding-prone microangiopathy. This hypothesis is further supported by the association with cerebral amyloid angiopathy (which is another causal disease for lobar hemorrhage) that is possibly of even greater importance: most amyloid angiopathy-related SMBs are found in the lobar area.
The integrity of the cerebral microvasculature is maintained by the blood.brain barrier and subtending basal lamina. Microvascular damage can result in extravasation of plasma and blood cells into brain parenchyma, and in overt hemorrhagic transformation. Considering that SMBs result from tiny extravasations of blood,6 it can be speculated that SMBs are associated with the formation of hemorrhagic transformation. Although microvascular structural disruption has not been directly associated with the development of SMBs, we believe that SMBs reflect the degree of bleeding tendency in the brain, and are predictive of subsequent bleeding episodes. Thus, the cerebral cortex of our patient might have been in a bleeding-prone state, as indicated by the presence of SMBs exclusively in the cortex. The subsequent embolic infarctions involved the deep gray matter and the cerebral cortex, but hemorrhagic conversion was observed only in the cortical infarction. Therefore, this case report indicates that the bleeding tendency may differ even within the same brain, and that this variation may be detected by analyzing the locations of SMBs.
It is possible that the cortical lesion in our patient was not a hemorrhagic conversion of embolic infarction but a pure hemorrhage. In DWI the lesion was surrounded by high signal intensities indicating cytotoxic edema. The concept of perihematomal ischemia or penumbra is not generally accepted,9 and high signal intensities are not usually observed in patients with intracerebral hemorrhage.10 A surrounding high signal on DWI may simply represent a paramagnetic artifact caused by a hematoma. However, the wide extents of high signals on DWI in our patient suggest that they were due to cytoxic edema associated with acute infarction, and did not originate from signal distortion due to extravasation of heme.
To our knowledge this is the first case report of a direct association between SMBs and the hemorrhagic conversion of a subsequent embolic infarction. Our observations suggest that SMBs are indicators of an underlying hemorrhage-prone state.
Figures and Tables
Figure 1
Magnetic resonance imaging (MRI) obtained before (A and B) and after (C and D) balloon angioplasty and stenting performed on both carotid arteries. The gradient-echo (GRE) sequence MRI image obtained before the stenting contained two silent microbleeds (SMBs, arrowheads) in the right parietal cortex (A), but no such lesions in the deep gray matter (B). After the stenting, embolic infarctions (arrows) were simultaneously present in two areas: the left striatum and right parietal cortex (C). However, a hemorrhagic conversion (arrowhead) was identified only around the cortical stroke lesions (D) where SMBs were originally present.
References
1. Lee SH, Bae HJ, Kwon SJ, Kim H, Kim YH, Yoon BW, et al. Cerebral microbleeds are regionally associated with intracerebral hemorrhage. Neurology. 2004. 62:72–76.

2. Fan YH, Zhang L, Lam WW, Mok VC, Wong KS. Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhages among patients with acute ischemic stroke. Stroke. 2003. 34:2459–2462.

3. Derex L, Nighoghossian N, Hermier M, Adeleine P, Philippeau F, Honnorat J, et al. Thrombolysis for ischemic stroke in patients with old microbleeds on pretreatment MRI. Cerebrovasc Dis. 2004. 17:238–241.

4. Lee SH, Bae HJ, Yoon BW, Kim H, Kim DE, Roh JK. Low concentration of serum total cholesterol is associated with multifocal signal loss lesions on gradient-echo magnetic resonance imaging: analysis of risk factors for multifocal signal loss lesions. Stroke. 2002. 33:2845–2849.

5. Lee SH, Park JM, Kwon SJ, Kim H, Kim YH, Roh JK, et al. Left ventricular hypertrophy is associated with cerebral microbleeds in hypertensive patients. Neurology. 2004. 63:16–21.

6. Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999. 20:637–642.
7. Lee SH, Kwon SJ, Kim KS, Yoon BW, Roh JK. Cerebral microbleeds in patients with hypertensive stroke. Topographical distribution in the supratentorial area. J Neurol. 2004. 251:1183–1189.

8. Lee SH, Bae HJ, Ko SB, Kim H, Yoon BW, Roh JK. Comparative analysis of the spatial distribution and severity of cerebral microbleeds and old lacunes. J Neurol Neurosurg Psychiatry. 2004. 75:423–427.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download