This article has been corrected. See "ERRATUM: Cognitive Impairment in Parkinson's Disease without Dementia: Subtypes and Influences of Age" in Volume 5 on page 202.
Abstract
Background and purpose
Chemokines participate in the regulation of immune and inflammatory responses by interacting with their receptors, which are primarily expressed on immune and inflammatory cells such as B- and T-lymphocytes and antigen-presenting cells. Chemokines and their receptors are therefore considered to mediate inflammation and tissue damage in autoimmune disorders. Chemokine receptor (CCR) genotypes were recently identified, and the importance of their genetic polymorphisms in some autoimmune and infectious disorders has been demonstrated. To define the roles of the polymorphism of the CCR2 gene at codon 64 (CCR2-64I) and the 32-bp deletion in the coding region of CCR5 (CCR5Δ32) in Korean patients with myasthenia gravis (MG), we compared these genotypes in MG cases and healthy controls and investigated the clinical features associated with these genotypes.
Methods
One hundred and fifteen healthy controls (51 men and 64 women) and 109 MG patients (44 men and 65 women) from three University hospitals were included. We examined each patient for clinical features using electrophysiology tests, laboratory tests, and thymic pathology. The CCR2-64I and CCR5Δ32 polymorphisms were determined by the PCR-RFLP method.
Results
We detected no difference in the frequencies of CCR2-64I polymorphism between MG patients and healthy controls. All of the MG patients and the healthy controls were homozygous for the wild-type CCR5 genotype. The results of electrophysiological tests and thymic pathologies were not influenced by the type of CCR2-64I polymorphism. However, the anti-acetylcholine-receptor (AChR) antibody titer was higher in the CCR2 G/G genotype (13.34±12.71 nmol/L) than in the CCR2 A/A genotype (5.83±2.56 nmol/L).
Myasthenia gravis (MG) is a T-cell-regulated, antibody-mediated autoimmune disorder. Although its clinical features and diagnostic methods are fairly well established, the underlying genetic factors for the elicitation and progression of MG are relatively unknown. A better understanding the molecular and cellular mechanisms underlying MG would improve disease management and help in the development of specific immunotherapies for MG.1 Many studies on MG patients have focused on the polymorphisms of immunoregulating factors, such as IL-1,2 TNF-α,3 CTLA-4,4 HLA subtypes,5 IgG receptors,6 the deltasubunit of the muscle acetylcholine receptor gene,7 and AChR epsilon.8 However, the results from these studies have been inconsistent, and they have not uncovered the etiopathology of MG.
Chemokines are a type of chemoattractant cytokines that direct the migration of leukocytes during inflammation and organize their homing into relevant organs.9 Chemokine receptors (CCRs) are G protein-coupled receptors that are primarily expressed on immune and inflammatory cells, such as B- and T-lymphocytes and antigen-presenting cells.10 Because chemokines and their receptors play roles in various inflammatory and autoimmune diseases, many investigators have focused on the associations between the pathophysiology of diseases, chemokines, and CCRs. Recent studies on rheumatoid arthritis, asthma,11 experimental autoimmune encephalomyelitis,12 and multiple sclerosis13 have all provided evidence supporting such associations. Furthermore, some studies have suggested that chemokines or CCRs affect the pathogenesis of MG.1,14
The G-to-A single-nucleotide polymorphism at position 190 of the CCR2 gene causes a change from valine to isoleucine at codon 64 (CCR2-64I) in the first transmembrane region of the protein. A 32-bp deletion in the coding region of CCR5 (CCR5Δ32) induces a premature stop codon in the third extracellular domain of the protein. Both of these polymorphisms have been associated with inflammatory diseases. The CCR2-64I allelic frequency is lower in patients with pulmonary sarcoidosis compared to controls, while the frequency of the CCR5Δ32 is significantly increased in the same population, suggesting specific roles for these polymorphisms in disease protection and susceptibility.15 Moreover, the CCR5Δ32 polymorphism was associated with multiple sclerosis in an HLA-DR4-positive Russian population, suggesting that CCR5Δ32 increases the risk of disease development.13 Based on these studies, we compared the distributions of polymorphic variants of these receptors in MG patients and aged-matched controls, to determine whether the CCR2-64I and CCR5Δ32 polymorphisms influence MG susceptibility.
One hundred and nine Korean patients (44 men and 65 women; mean age±SD=42.8±15.2 years) with MG were recruited from three university hospitals and signed written informed consents. All patients underwent a standard battery of examinations, including medical history, physical and neurological examinations, and laboratory screening tests (including autoimmune screening tests and thyroid function tests). The diagnostic criteria for MG were the presence of characteristic clinical features with at least one positive finding from among the serum anti-AChR antibody test, neostigmine test, and repetitive nerve stimulation test. The control group consisted of 115 age- and sex-matched healthy subjects (51 men and 64 women; mean age±SD=43.4±10.0 years) who visited the Health Promotion Center of Yongdong Severance hospital for regular health checkups. All control subjects signed written informed consents and underwent the standard battery of laboratory and physical examinations. Control subjects with any evidence of neurological diseases including MG or autoimmune disease were excluded.
The frequencies of alleles and genotypes were obtained by direct counting. Pearson's chi-square test was used to determine the statistical significance of differences in genotypes and haplotypes between the control and patient groups. Pearson's chi-square test was also used to analyze differences in the neurophysiological test results (including the repetitive nerve stimulation test, neostigmine test, and single-fiber EMG), thymic pathologies, and clinical features according to the genotypes of the patients. A oneway analysis of variance (ANOVA) test was used to test for significant differences in the anti-AChR antibody titers between patients. Statistical analysis was performed using SPSS software (Version 11.0), and the cutoff for statistical significance was set at p<0.05 (two-sided).
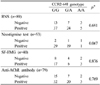
The mean ages at MG onset and diagnosis were 37.5±17.1 and 38.9±17.1 years, respectively. Ocular symptoms (ptosis and diplopia) were most common at the initial stage (64 cases, 58.7%) and during the diagnostic period (53 cases, 48.6%). The symptoms (at the initial and diagnostic periods) and clinical classifications (according to the classifications of the Myasthenia Gravis Foundation of America and Osserman) did not differ significantly with the CCR2-64I genotype (Table 1).
The CCR2-64I G/G, G/A, and A/A genotypes occurred in 61 (56.0%), 37 (33.9%), and 11 (10.1%) MG patients, respectively; in 68 (59.1%), 41 (35.7%), and 6 (5.2%) healthy controls (p=0.387). The allele frequencies also did not differ between the MG patients and the control group (p=0.972) (Table 2). For the CCR5Δ32 genotype, all of the MG patients and the controls were homozygous for the wild type.
We performed the repetitive nerve stimulation test in 89 patients, the neostigmine test in 53 patients, and single-fiber EMG in 40 patients. The results of these electrophysiological tests were not influenced by the type of CCR2-64I polymorphism (Table 3). The anti-AChR antibody titer was measured in 79 patients, in whom the frequencies of seronegative and seropositive patients did not differ with the type of CCR2-64I polymorphism. There were no statistically significant differences between anti-AChR antibody titers in patients with the G/G (13.34±12.71 nmol/L), G/A (8.18±4.67 nmol/L), and A/A (5.83±2.56 nmol/L) genotypes (ANOVA, p=0.148). However, the anti-AChR antibody titer was higher in patients with the G/G genotype than in those with the A/A genotype (ANOVA, post-hoc analysis, p=0.038). Among 48 patients who underwent thymectomy, 15 patients exhibited normal pathology, 16 patients had thymic hyperplasia, and 17 patients had a thymoma. The thymic pathology did not differ with the type of CCR2-64I polymorphism (p=0.087).
Basic molecular aspects of chemokine biology have been found to differ with race.16 While the frequencies of the CCR5Δ32 allele are high in northern European populations (11~15%), these frequencies are very low in Greece, Saudi Arabia, Pakistan, and India (1~3%).17,18 Furthermore, the CCR5Δ32 allele was not been found in China, Japan, Korea, or Thailand.18,19 In contrast, the CCR2-64I allele has been found in almost all studied populations and is more frequent in Africa and Asia than in northern Europe.20 Immigration, genetic admixtures, and the founder effect might be responsible for these CCR polymorphic differences. The frequency of the CCR2-64I allele in our Korean population (25%) is similar to that previously reported for Japanese (24%).17 The frequency of the CCR5Δ32 polymorphism in the present study is also consistent with previous report.19
The relationship between proinflammatory cytokines and chemokines in MG can be inferred from previous reports. The cytokine, interferon-γ (IFN-γ) significantly influences the symptoms and progression of experimental autoimmune MG (EAMG), and IFN-γ regulated genes are highly expressed in the thymic tissues of MG patients.21,22 In addition, IFN-γ and TNF-α both upregulate AChR transcripts and thereby contribute to the initiation of the autoimmune anti-AChR response.21 IFN-γ or TNF-α increase the expressions of monocyte chemotactic protein-1 (MCP-1) and RANTES (regulated on activation, normal T-cell expressed and secreted).23 MCP-1 is a potent and specific monocyte agonist and chemoattractant.24 Mononuclear cells infiltrate thymus and skeletal-muscle EAMG, and other studies have also shown increased CCR5-positive mononuclear cells in chronically inflamed tissues.25,26 Increased MCP-1 production may explain the increased leukocyte trafficking through muscle following antibody transfer and induction of MG symptoms in EAMG.27 It is postulated that MCP-1 and RANTES play a central role in those cytokine cascade. Because MCP-1 binds to CCR2 and RANTES binds to CCR5 with high affinity,24,28 the CCR2 and CCR5 polymorphisms might be important genetic factors for MG initiation and progression.
CCR2 is believed to mediate blood monocyte extravasation to sites of inflammation and be involved in macrophage recruitment to the injured peripheral system.29 The frequency of homozygotes for CCR2-64I was found to be lower in sarcoidosis and Alzheimer's disease than in controls, thus indicating that the wildtype CCR2 (G/G genotype) could be associated with greater inflammation in autoimmune inflammatory disease.15,30 Moreover, an animal study has suggested that IFN-γ-induced MCP-1 contributes to MG progression and that CCR2 is involved in disease progression or disease induction.27
In our study, we found no evidence of an increased risk for MG in the genotypes of CCR2-64I polymorphism. A previous study also found that the frequencies of CCR2 and CCR5 polymorphisms did not differ between the MG patients and controls.10 Although reports have suggested that CCR2 is involved in disease progression,27,29 we found that the clinical features and electrophysiological tests in MG patients did not differ with the type of CCR2-64I polymorphism. Interestingly, the anti-AChR antibody titer was higher in the G/G genotype than in the A/A genotype, even though the presence of this antibody did not differ with the type of CCR2-64I polymorphism. Previous studies have suggested that proinflammatory cytokines contribute to the initiation of autoimmune anti-AChR responses,21 and upregulated MCP-1 production by the skeletal muscle was associated with the presence of circulating anti-AChR antibodies.27 Although it has been widely accepted that antibody titer and disease severity or progression in MG are not quantitatively related,31 our results support the hypothesis that CCR2 plays a role in MG pathophysiology via the production of anti-AChR antibodies. In MG patients, cytokine activity is suggested to initiate the autoimmune anti-AChR response.21 Moreover, by upregulating chemokines, anti-AChR antibodies may be able to influence the severity and course of experimental MG.27 We propose that larger studies involving measurements of the levels of related cytokines will precisely identify the clinical and pathophysiologic relationship between CCR2 and anti-AChR antibody.
Figures and Tables
References
1. Feferman T, Maiti PK, Berrih-Aknin S, Bismuth J, Bidault J, Fuchs S, et al. Overexpression of IFN-induced protein 10 and its receptor CXCR3 in myasthenia gravis. J Immunol. 2005. 174:5324–5331.

2. Huang D, Pirskanen R, Hjelmstrom P, Lefvert AK. Polymorphisms in IL-1beta and IL-1 receptor antagonist genes are associated with myasthenia gravis. J Neuroimmunol. 1998. 81:76–81.

3. Huang DR, Pirskanen R, Matell G, Lefvert AK. Tumour necrosis factor-alpha polymorphism and secretion in myasthenia gravis. J Neuroimmunol. 1999. 94:165–171.
4. Huang D, Liu J, Noren K, Xia SQ, Trifunovic J, Pirskanen R, et al. Genetic association of Ctla-4 to myasthenia gravis with thymoma. J Neuroimmunol. 1998. 88:192–198.

5. Vieira MA, Caillat-Zucman S, Gajdos P, Cohen-Kaminsky S, Casteur A, Bach JF. Identification by genomic typing of non-DR3 HLA class II gene associated with myasthenia gravis. J Neuroimmunol. 1994. 47:115–122.

6. van der Pol WL, Jansen MD, Kuks JB, de Baets M, Leppers-van de Straat FG, Wokke JH, et al. Association of the Fc gamma receptor IIA-R/R131 genotype with myasthenia gravis in Dutch patients. J Neuroimmunol. 2003. 144:143–147.

7. Giraud M, Eymard B, Tranchant C, Gajdos P, Garchon HJ. Association of the gene encoding the delta-subunit of the muscle acetylcholine receptor (CHRND) with acquired autoimmune myasthenia gravis. Genes Immun. 2004. 5:80–83.

8. Bonifati DM, Willcox N, Vincent A, Beeson D. Lack of association between acetylcholine receptor epsilon polymorphisms and early-onset myasthenia gravis. Muscle Nerve. 2004. 29:436–439.

9. Luster AD. Chemokines - chemotactic cytokines that mediate inflammation. N Engl J Med. 1998. 338:436–445.

10. Zhao X, Gharizadeh B, Hjelmstrom P, Pirskanen R, Nyren P, Lefvert AK, et al. Genotypes of CCR2 and CCR5 chemokine receptors in human myasthenia gravis. Int J Mol Med. 2003. 12:749–753.

11. Strieter RM, Standiford TJ, Huffnagle GB, Colletti LM, Lukacs NW, Kunkel SL. "The good, the bad, and the ugly." The role of chemokines in models of human disease. J Immunol. 1996. 156:3583–3586.
12. Miyagishi R, Kikuchi S, Takayama C, Inoue Y, Tashiro K. Identification of cell types producing RANTES, MIP-1 alpha and MIP-1 beta in rat experimental autoimmune encephalomyelitis by in situ hybridization. J Neuroimmunol. 1997. 77:17–26.

13. Favorova OO, Andreewski TV, Boiko AN, Sudomoina MA, Alekseenkov AD, Kulakova OG, et al. The chemokine receptor CCR5 deletion mutation is associated with MS in HLA-DR4-positive Russians. Neurology. 2002. 59:1652–1655.

14. Saito R, Onodera H, Tago H, Suzuki Y, Shimizu M, Matsumura Y, et al. Altered expression of chemokine receptor CXCR5 on T cells of myasthenia gravis patients. J Neuroimmunol. 2005. 170:172–178.

15. Petrek M, Drabek J, Kolek V, Zlamal J, Welsh KJ, Bunce M, et al. CC chemokine receptor gene polymorphisms in Czech patients with pulmonary sarcoidosis. Am J Respir Crit Care Med. 2000. 162:1000–1003.

16. Wang FS, Hong WG, Cao Y, Liu MX, Jin L, Hu LP, et al. Population survey of CCR5 delta32, CCR5 m303, CCR2b 64I, and SDF1 3'A allele frequencies in indigenous Chinese healthy individuals, and in HIV-1-infected and HIV-1-uninfected individuals in HIV-1 risk groups. J Acquir Immune Defic Syndr. 2003. 32:124–130.

17. De Pinho Lott Carvalhaes FA, Cardoso GL, Hamoy IG, Liu YT, Guerreiro JF. Distribution of CCR5-delta32, CCR2-64I, SDF1-3'A mutations in populations from the Brazilian Amazon Region. Hum Biol. 2004. 76:643–646.
18. Gharagozloo M, Doroudchi M, Farjadian S, Pezeshki AM, Ghaderi A. The frequency of CCR5Delta32 and CCR2-64I in southern Iranian normal population. Immunol Lett. 2005. 96:277–281.

19. Oh MD, Kim SS, Kim EY, Lee S, Kim N, Park KY, et al. The frequency of mutation in CCR5 gene among Koreans. Int J STD AIDS. 2000. 11:266–267.

20. Martison JJ, Hong L, Karanicolas R, Moore JP, Kostrikis LG. Global distribution of the CCR2-64I/CCR5-59653T HIV-1 disease-protective haplotype. AIDS. 2000. 14:483–489.

21. Poea-Guyon S, Christadoss P, Le Panse R, Guyon T, De Baets M, Wakkach A, et al. Effects of cytokines on acetylcholine receptor expression: implications for myasthenia gravis. J Immunol. 2005. 174:5941–5949.

22. Shi FD, Zhang GX, Bai XF, Van der Meide PH, Link H. Cellular mRNA expression of interferon-gamma (IFN-gamma), IL-4 and IL-10 relates to resistance to experimental autoimmune myasthenia gravis (EAMG) in young Lewis rats. Clin Exp Immunol. 1997. 108:523–528.

23. Reyes-Reyna S, Krolick KA. Chemokine production by rat myocytes exposed to interferon-gamma. Clin Immunol. 2000. 94:105–113.
24. Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci U S A. 1994. 91:2752–2756.

25. Gu D, Wogensen L, Calcutt NA, Xia C, Zhu S, Merlie JP, et al. Myasthenia gravis-like syndrome induced by expression of interferon gamma in the neuromuscular junction. J Exp Med. 1995. 181:547–557.

26. Rottman JB, Ganley KP, Williams K, Wu L, Mackay CR, Ringler DJ. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am J Pathol. 1997. 151:1341–1351.
27. Reyes-Reyna S, Stegall T, Krolick KA. Muscle responds to an antibody reactive with the acetylcholine receptor by up-regulating monocyte chemoattractant protein 1: a chemokine with the potential to influence the severity and course of experimental myasthenia gravis. J Immunol. 2002. 169:1579–1586.

29. Siebert H, Sachse A, Kuziel WA, Maeda N, Bruck W. The chemokine receptor CCR2 is involved in macrophage recruitment to the injured peripheral nervous system. J Neuroimmunol. 2000. 110:177–185.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download