Abstract
Lymphomatoid granulomatosis (LG) is a potentially malignant lymphoproliferative disorder. The lung is the most common involved site, followed by the skin and nervous system. However, LG of the central nervous system presenting with Parkinsonism is very rare. We report a patient with LG who presented with parkinsonian features such as bilateral rigidity, bradykinesia, and agitation. Brain magnetic resonance imaging showed multifocal punctuate enhanced lesions in both supra- and infratentorial areas. Steroid pulse therapy resulted in a dramatical improvement in the symptoms and MRI abnormalities.
Lymphomatoid granulomatosis (LG) is an atypical, potentially malignant lymphoproliferative disorder that mainly involves the lung, followed by the skin and the brain.1 The central nervous system (CNS) is involved in up to one-third of LG patients. However, isolated CNS involvement is rare,2,3 and its clinical features are variable. We report a patient who presented with subacute onset of symmetric Parkinsonism associated with LG involving the CNS.
A 21-year-old man was admitted for the evaluation of leukopenia and thrombocytopenia. He was diagnosed with lymphoblastic lymphoma and treated with a chemotherapeutic regimen (Hyper-CVAD). Ten days after the chemotherapy, he became agitated and exhibited temporary whole-body tremor that made him unable to stand still. On examination, he appeared dull and apathetic, and his gait was slow and characterized by short steps, shuffling, the paucity of arm swing, and difficulty in turning of the body. His posture was stooped forward, and he complained of a sense of heaviness and clumsiness in his activities of daily living. His family noticed that he took a long time to respond to questions. There were bilateral mild-to-moderate postural and action tremors, symmetric and moderate rigidity with bradykinesia, and moderately shuffling gait in a stooped posture according to the Unified Parkinson's Disease Rating Scale (UPDRS).4 Laboratory findings were inconclusive. Cerebrospinal fluid (CSF) findings were as followed: pleocytosis (90 WBCs/mm3, 70% mononuclear cells), no red blood cells, mild elevation of protein (51.9 mg/dL), and normal glucose (72 mg/dL). Neither microorganisms nor malignant lymphocytes were detected in the CSF. PCR of CSF samples for mycobacterium tuberculosis and an antibody against fungus produced negative results.
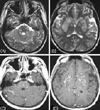
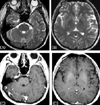
Radiologic searches for systemic lesions related with LG showed no abnormalities. T2-weighted axial MRI showed numerous disseminated hyperintense lesions in the whole brain, mainly in both basal ganglia, thalamus, cerebral cortex, white matter, brainstem, and cerebellum. The lesions showed multifocal punctuated enhancement with gadolinium (Fig. 1). We used a steroid to treat LG because the patient and his family did not allow us to perform a brain biopsy. Pulse therapy with methylprednisolone (1000 mg/day) for 5 days resulted in most of the disseminated lesions disappearing in follow-up brain MRI, and they had completely resolved 2 months later (Fig. 2).
Whilst mild shuffling of the gait remained (mild severity according to the UPDRS4), the parkinsonian features in the limbs of the patient such as tremor, rigidity, and bradykinesia were no longer present after the therapy. His facial expression was appropriate and his speed of thought processing was improved. Cognitive evaluation using the Korean version of Wechsler Adult Intelligence Scale (KWAIS) had initially showed a lower-than-average IQ and dissociation between verbal and performance IQ. Two months later, he showed better cognitive function and an average IQ on the KWAIS.
We experienced a patient who presented only with subacute-onset symmetric Parkinsonism associated with disseminated multifocal enhanced lesions in the brain. He had been previously diagnosed with lymphoblastic lymphoma and treated with Hyper-CVAD. Multiple disseminated lesions in the subcortical gray matter, including the basal ganglia and thalamus, might have been responsible for the Parkinsonism and slowing of thought processing in this patient.
CNS complications of hematologic neoplasm are caused either by the primary disease or by the effects of therapy.5 LG is one of the rare diseases that causes CNS complications in patients with hematologic neoplasm. Multifocal gadolinium-enhanced lesions in the brain can be caused by infiltration of primary hematologic disease or opportunistic infection with disseminated tuberculosis or fungus, so clinicians should differentiate LG and infectious causes. Neither malignant cells nor microorganisms in the CSF were found or grown in the present case. Also, pulse therapy with a steroid was effective for the parkinsonian symptoms and the brain lesions without the use of antituberculous and antifungal agents. The known CNS complications of Hyper-CVAD are only related to steroids,6 and hence the present subject represents a novel case. MRI is more sensitive than CSF cytologic analysis or flow cytometry for detecting CNS involvement from LG.7 Therefore, we considered the CNS lesion in this case to be LG since there was no evidence of opportunistic infections or complications of chemotherapy, and the patient showed specific MRI findings of LG and a good response to steroid therapy. Despite these findings, we were unable to make a definitive diagnosis due to the lack of pathologic data.
LG presents diverse neuroimaging features, such as multiple punctuate or linear enhancements, ring-like enhancements, large enhanced mass lesions, leptomeningeal involvement, and choroid plexus involvement.7,8 Disseminated multifocal enhancement in the present case is a typical pattern of angiocentric involvement with LG. The clinical features of its CNS involvement also are variable, including headache, seizure, blindness, cranial nerve palsies, hemiparesis, ataxia, spastic gait, dementia, and altered consciousness.2,3 Parkinsonism is a rare presenting symptom of LG.9
Parkinsonism could be the only presenting manifestation of CNS involvement of LG, and it is worth applying steroid therapy in cases with clinical suspicion because the prognosis of LG involved CNS is variable, ranging from spontaneous resolution to rapid fatality.10
Figures and Tables
Figure 1
Brain MRI performed before treatment with steroid. T2-weighted MRI showed disseminated and multifocal hyperintense lesions in both basal ganglia, thalamus, cerebral cortex, and white matter (A, B), and disseminated multiple punctuate enhanced lesions in the corresponding areas of the T1-weighted images with gadolinium enhancement (C, D).
References
2. Bae WK, Lee KS, Kim PN, Kim IY, Lee NH, Lee KS, et al. Lymphomatoid granulomatosis with isolated involvement of the brain. J Korean Med Sci. 1991. 6:255–259.

3. Paspala AB, Sundaram C, Purohit AK, Immaneni D. Exclusive CNS involvement by lymphomatoid granolomatosis in a 12-year-old boy: a case report. Surg Neurol. 1999. 51:258–260.

4. Herndon RM. Handbook of neurologic rating scales. 2006. 2nd edn. New York: Demos;145–155.
5. Vazquez E, Lucaya J, Castellote A, Piqueras J, Sainz P, Olive T, et al. Neuroimaging in pediatric leukemia and lymphoma: differential diagnosis. Radiographics. 2002. 22:1141–1428.

6. Kantarjian H, Thomas D, O'Brien S, Cortes J, Giles F, Jeha S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regiment, in adult acute lymphocytic leukemia. Cancer. 2004. 101:2788–2801.

7. Patsalides AD, Atac G, Hedge U, Janik J, Grant N, Jaffe ES, et al. Lymphomatoid granulomatosis: abnormalities of the brain at MR imaging. Radiology. 2005. 237:265–273.

8. Tateishi U, Terae S, Ogata A, Sawamura Y, Suzuki Y, Abe S, et al. MR imaging of the brain in lymphomatoid granulomatosis. Am J Neuroradiol. 2001. 22:1284–1290.
9. Oliveras C, D'Olhaberriague L, Garcia J, Matias-Guiu X. Parkinsonism as first manifestation of lymphomatoid granulomatosis. J Neurol Neurosurg Psychiatry. 1988. 51:999–1001.

10. Jaffe ES, Wilson WH. Lymphomatoid granulomatosis: pathogenesis, pathology and clinical implications. Cancer Surv. 1997. 30:233–248.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download