Abstract
Truncal contrapulsion in association with pretectal syndrome has not been described previously. We report a patient with vertical-gaze palsy and severe truncal contrapulsion due to an infarction in the mesodiencephalic junction. Truncal contrapulsion in this patient may have resulted from the disruption of the ascending fibers in the crossed cerebellothalamic tract.
The pretectum lies in the prerubral field in the mesodiencephalic junction and includes several structures that are involved in the control of eyelid and vertical eye motion. Lesions involving the pretectum (i.e., pretectal syndrome) usually present with vertical-gaze palsy, convergence-retraction oscillation of the eyes, lid retraction, and light-near dissociation of the pupils.1 Truncal lateropulsion refers to the falling to one side that cannot be explained by weakness or limb ataxia. Truncal ipsipulsion has been described primarily in Wallenberg syndrome, and may occur with lesions that involve the peripheral vestibular system, brainstem, cerebellum, or basal ganglia.2 Truncal contrapulsion has been reported in both pontomesencephalic and thalamic lesions.3 However, truncal contrapulsion in pretectal syndrome has not been described previously. We report a case of pretectal syndrome and truncal contrapulsion caused by a mesodiencephalic infarction.
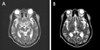
A 74-year-old man presented with sudden dizziness and falling to the right side. He also noted a droopy eyelid on the left side. He stated that associated nausea, vomiting, and eyeball pain were not present. He had been hypertensive for 30 years and had a 30-pack-year smoking history. His blood pressure was 130/80 mmHg and his heart rate was 78 beats/minute. The patient was drowsy; however, he was able to cooperate during the neurological examination. He showed complete ptosis of the left eye and mild lid retraction of the right eye (Fig. 1). Manual opening of the left eye did not result in resolution of right lid retraction, which suggested true rather than compensatory retraction to overcome left-eye ptosis. The right pupil was reactive to light and accommodative stimuli. The left pupil was dilated to 5 mm without reflexes. In the left eye, only abduction was possible with complete impairments of adduction, elevation, and depression. In the right eye, abduction and adduction were both normal. However, the elevation (<25% of the normal range) and depression (<50% of the normal range) of the right eye were both limited. The function of the left trochlear nerve was determined to be normal from intorsion of the left eye on attempted downward gaze. No ocular retraction was noted on attempted vertical gaze. The patient had mild right hemiparesis of MRC grade IV. He could not even sit without support due to rightward falling. Limb ataxia was not present.
An electrocardiogram and complete blood counts and chemistry did not reveal any abnormal findings. Diffusion MRI revealed an acute infarction in the left medial midbrain that extended to the left medial thalamus (Fig. 2). MR angiography revealed mild stenoses in both the left proximal posterior cerebral and right distal vertebral arteries. Findings from two dimensional- and transesophageal echocardiography were normal. The patient was presumed to have suffered from an "artery-to-artery embolism" that originated in the right distal vertebral artery and was subsequently treated with anticoagulation drugs. The right hemiparesis improved markedly over the following 2 weeks. However, ophthalmoplegia and truncal contrapulsion remained unchanged at 1 month after the initial presentation.
Our patient presented with characteristic ocular motor findings that could have been due to the lesion involving both the pretectum and upper midbrain.4 Among the symptoms of our patient, ophthalmoplegia in the left eye may have been due to the lesion that involved either the oculomotor fascicle or nucleus in the midbrain. However, involvement of the oculomotor nucleus may be excluded in view of the presence of unilateral ptosis. Accordingly, vertical ophthalmoplegia and lid retraction in the right eye required the additional involvement of supranuclear structures involved in vertical gaze and eyelid function in the mesodiencephalic junction.
The mesodiencephalic junction contains the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF), the interstitial nucleus of Cajal (INC), the rostral portion of the mesencephalic reticular formation (MRF), and the posterior commissure (PC), all of which are involved in the premotor control of vertical eye movements. The riMLF lies in the prerubral area near the midline and contains medium-lead burst neurons that generate vertical saccades.5 Inactivation of the riMLF in monkeys has been shown to reduce the velocity or completely abolish conjugate vertical saccades.6 The INC, together with vestibular nuclei, is an element of the neural integrator for vertical eye motion, which transforms vertical eye velocity signals to position signals.7 The rostral portion of the MRF, adjacent to the INC and riMLF, contains neurons that have low -frequency, long-lead burst activity prior to vertical saccades.8 Unilateral inactivation of the rostral MRF produces slowed and hypometric upward and downward saccades without postsaccadic drift.9 The PC contains several groups of decussating axons from the adjacent nucleus of the PC (NPC) and axons from both the riMLF and INC that project to corresponding structures in the contralateral midbrain tegmentum.10 The NPC also contains neurons that discharge shortly before upward saccades.11 The PC is critical for upward saccades.12
Our patient also showed mild lid retraction OD (right eye) and complete ptosis OS (left eye) that resembled the "plus-minus syndrome" that was reported previously in a thalamus-midbrain infarction. In patients with unilateral ptosis, the contralateral eyelid may appear retracted since excessive innervation (to overcome the ptosis) to the levator palpebra of the ptotic eye is delivered to the normal levator palpebra in the contralateral eye (i.e., Hering's law). In this case, manual elevation of the ptotic eyelid would result in resolution of lid retraction in the contralateral eye. In our patient, however, the persistence of lid retraction in the right eye even during manual opening of the left eye suggested true-lid retraction. The cortex, extrapyramidal motor systems, and rostral brainstem structures are known to contribute to the control of the levator palpebra muscle in various eyelid functions.13 PC and riMLF signals may be involved in lid-eye coordination by providing inhibitory modulation of levator palpebra motor activity. Lesions of the medial or principal portion of the PC nuclear complex appear to be essential for the production of lid retraction.13
Truncal lateropulsion is a well-known sign of Wallenberg syndrome and has rarely been described in either anterolateral thalamic or cingulated gyrus lesions.14,15 So-called thalamic astasia is believed to interrupt the projections that run from the ventrolateral nucleus of the thalamus to the medial portion of the precentral gyrus where the trunk and leg are represented. The involvement of the fastigial projections through which the thalamus receives information from the vestibulocerebellum is the presumed mechanism of thalamic astasia, even though the exact mechanism remains to be elucidated.3 Disruption of the connection between the cingulate motor area and the vestibulocerebellar system via the thalamic nuclei was assumed to underlie the truncal contrapulsion observed in a patient with posterior cingulate infarction.14 However, there have been no previous reports of truncal contrapulsion with medial thalamic lesions. In view of two cases of rubral lateropulsion that were not accompanied by cerebellar ataxia, we also postulate that the lateropulsion observed in our patient with a mesodiencephalic lesion was due to the disruption of the ascending fibers in the crossed cerebellothalamic tract.15 This explanation is also supported by previous reports that the vestibulothalamic, dentatorubrothalamic, and fastigiothalamic fibers join the thalamic fascicle adjacent to the red nucleus and that interruption of any of these fibers can cause lateropulsion.15 Frequent occurrences of axial lateropulsion (i.e., ipsipulsion) in patients with infarctions in the region of the medial superior cerebellar artery, which presumably damages the cerebellothalamic tract prior to decussation, also supports our hypothesis.16
Figures and Tables
References
1. Keane JR. The pretectal syndrome: 206 patients. Neurology. 1990. 40:684–690.
2. Thomke F, Marx JJ, Iannetti GD, Cruccu G, Fitzek S, Urban PP, et al. A topodiagnostic investigation on body lateropulsion in medullary infarcts. Neurology. 2005. 64:716–718.

3. Masdeu JC, Gorelick PB. Thalamic astasia: inability to stand after unilateral thalamic lesions. Ann Neurol. 1988. 23:596–603.

4. Sharpe JA, Kim JS. Midbrain disorders of vertical gaze: a quantitative re-evaluation. Ann N Y Acad Sci. 2002. 956:143–154.
5. King WM, Fuchs AF. Reticular control of vertical saccadic eye movements by mesencephalic burst neurons. J Neurophysiol. 1979. 42:861–876.

6. Suzuki Y, Büttner-Ennever JA, Straumann D, Hepp K, Hess BJM, Henn V. Deficits in torsional and vertical rapid eye movements and shift of Listing's plane after uni- and bilateral lesions of the rostral interstitial nucleus of the medial longitudinal fasciculus. Exp Brain Res. 1995. 106:215–232.

7. Fukushima K, Fukushima J, Harada C, Ohashi T, Kase M. Neuronal activity related to vertical eye movement in the region of the interstitial nucleus of Cajal in alert cats. Exp Brain Res. 1990. 79:43–64.

8. Handel A, Glimcher PW. Response properties of saccade-related burst neurons in the central mesencephalic reticular formation. J Neurophysiol. 1997. 78:2164–2175.

9. Waitzman DM, Silakov VL, DePalma-Bowles S, Ayers AS. Effects of reversible inactivation of the primate mesencephalic reticular formation. II. Hypometric vertical saccades. J Neurophysiol. 2000. 83:2285–2299.

10. Kokkoroyannis T, Scudder CA, Balaban CD, Highstein SM, Moschovakis AK. Anatomy and physiology of the primate interstitial nucleus of Cajal I. efferent projections. J Neurophysiol. 1996. 75:725–739.

11. Moschovakis AK, Scudder CA, Highstein SM. Structure of the primate oculomotor burst generator. I. Medium-lead burst neurons with upward on-directions. J Neurophysiol. 1991. 65:203–217.

12. Keane JR, Davis RL. Pretectal syndrome with metastatic malignant melanoma to the posterior commissure. Am J Ophthalmol. 1976. 82:910–914.

13. Schmidtke K, Büttner-Ennever JA. Nervous control of eyelid function. A review of clinical, experimental and pathological data. Brain. 1992. 115:227–247.
14. Kataoka H, Sugie K, Kohara N, Ueno S. Novel representation of astasia associated with posterior cingulated infarction. Stroke. 2006. 37:e3–e5.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download