Abstract
Background and purpose
Anxiety is the most important precipitating factor of migraine attacks, and more than half of migraineurs have coexisting anxiety disorders. Paroxetine, an antidepressant, is one of the selective serotonin reuptake inhibitors (SSRIs) that has an anxiolytic effect, and is also known to be effective for migraine prophylaxis. The aim of this study was to determine the role of the anxiolytic effect of paroxetine on the prevention of migraine.
Methods
This study investigated migraineurs with a general anxiety disorder who visited the neurological clinic. The following efficacy variables were assessed at baseline and after taking paroxetine (20 for 12 weeks: headache frequency, Hamilton Anxiety Rating Scale (HAM-A), Headache Management Self-Efficacy Scale (HMSE), and Headache Disability Inventory (HDI). The correlation between the headache responsiveness to paroxetine and improvement in anxiety levels was analyzed.
Results
Twenty-four patients (aged 54.96±12.09 years, mean±SD) were included in this study. Paroxetine reduced headache frequency by 49.1% within 12 weeks (p<0.05 vs baseline). HAM-A and HMSE scores also showed an improvement, whereas there was no significant change in HDI score. The baseline HAM-A scores did not differ between paroxetine responders and nonresponders. In addition, the improvement in HAM-A score was not correlated with the reduction in headache frequency.
Conclusions
Paroxetine decreased the headache frequency and reduced anxiety levels. However, the anxiolytic effect of paroxetine was not correlated with the migraine prevention effect. These observation indicate that the anxiolytic effect of paroxetine does not contribute strongly to its prophylactic effect on migraine frequency in migraineurs with anxiety disorder.
Migraine is a common neurologic disorder, with reported prevalences of 10-12% in Western countries1 and 8.4-22.3% in Eastern countries.2-4 Anxiety is one of the most important precipitating factors of migraine attacks and is commonly observed during prodrome or the migraine attack itself. The comorbidity rate of migraine and anxiety disorder has been reported in some studies to be more than 50%.5-7
Antidepressants such as tricyclic antidepressants (TCAs), serotonin reuptake inhibitors (SSRIs), and serotonin norepinephrine reuptake inhibitors (SNRIs) exhibit some prophylactic effects against migraine, but the underlying mechanisms have not been defined.8-10 Because antidepressants usually have an anxiolytic effect, it has been suggested that the improvement in anxiety is related with the prevention of a migraine attack.10 However, no previous study has shown that a migraine attack can be prevented by reducing anxiety levels.
Paroxetine, an SSRI, is used widely as an antidepressant or anxiolytic. While it has no GABAergic effects, it is 22 times more potent than fluoxetine, 7 times thansertraline, and 80-100 times than amitriptyline or imipramine, respectively. In this study we determined the preventive effect of paroxetine for migraine, and assessed whether the anxiolytic effect of paroxetine is correlated with its prophylactic effect against migraine.
This study was a prospective, open-label trial performed in a single center. Written approval to carry out the study was received from the Institutional Review Board, and all subjects gave their informed consent to participate before the study commenced. This study recruited migraineurs with anxiety disorder. The following inclusion criteria were applied: (1) a diagnosis of migraine without aura according to the criteria of the International Headache Society 2004,11 (2) conformance with the Diagnostic and Statistical Manual of Mental Disorders IV criteria for generalized anxiety disorder, (3) an initial Hamilton Anxiety Rating (HAM-A) score of 18 or more, (4) age over 18 years, (5) the provision of signed assent, and (6) the ability to read and understand the self-reporting scales used in this study.
Subjects with the following history of conditions were excluded: (1) previously treated with paroxetine or an SSRI, (2) hepatitis or renal disease, as indicated by threefold elevations in the normal upper limits of GOT, GPT or bilirubin creatinine level > 2.0 mg/dl (176.7μmol/l) or receiving dialysis, (3) cardiovascular diseases, (4) surgical operation that can affect drug absorption or renders the patient unable to take oral medication, (5) disability or deficit, as assessed by a physical or neurological examination, (6) history of psychiatric disease, (7) prophylactic migraine medication that can influence a migraine attack within 4 weeks, (8) alcoholism or history of drug addiction, and (9) history of allergy or hypersensitivity to paroxetine.
Paroxetine was administered at a dosage of 20 in two divided doses for 12 weeks. The dosage was halved to 10 mg twice a day if the subject reported side effects. If side effects persisted, the subject was withdrawn from the trial.
During the initial visit, patients were screened and underwent a complete evaluation of their medical history, and laboratory testing. After a 4-week baseline period, patients were followed up every 4 weeks for up to 12 weeks. During this follow-up period, all patients completed a headache diary and other scales, from which efficacy scales such as headache frequency were calculated. Mean headache frequencies were monitored every 4 weeks. The responder rate, which was the primary efficacy outcome, was defined as the percentage of subjects showing a reduction of 50% or greater in the attack frequency at 3 months compared to the baseline headache frequency. The HAM-A,12 Headache Disability Inventory (HDI),13 and the Headache Management Self-Efficacy (HMSE) scale were assessed at every visit.14,15 Secondary efficacy outcomes included changes in headache frequency and in the HAM-A score.
To minimize possible bias from the withdrawal of subjects from the study, the final observation of apatient that discontinued the study was carried forward to all subsequent assessment periods (last observation carried forward, LOCF). Comparisons of headache frequency, HAM-A, and HMSE scores to baseline values were performed using the paired t-test. The correlation between headache frequency and HAM-A score was analyzed by bivariate correlation analysis. Statistical significance was accepted for probability values of p<0.05.
Twenty-four consecutive migraineurs who met the criteria for general anxiety disorders were included in this study (see Table 1). They were aged between 33 and 74 years (54.9±12.1 years, mean±SD). The patients comprised 8 men and 16 women, and the mean age did not differ significantly with gender (males 51.6±15.1 years, females 56.8±10.0 years; p=0.223). Two patients withdrew from the study at the second visit (at 2 months) and one withdrew at the third visit (at 3 months).
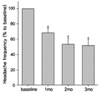
The headache frequencies at baseline and at 1, 2, and 3 months after treatment were 18.4±12.1, 15.2±12.1, 13.8±13.0, and 13.3±13.1 attacks per month, respectively. When the headache frequencies of each individual are expressed as percentage ratios relative to their own baseline headache frequencies, the mean headache frequencies at 1, 2, and 3 months after treatment were 68.4±31.7%, 53.7±41.2%, and 51.9±41.7%, respectively (all p<0.01 vs baseline, paired t-test; Table 1 and Fig. 1). Accordingly, paroxetine induced a 48.1% reduction in headache frequency at 3 months compared to the baseline.
Paroxetine treatment resulted in an improvement in mean HAM-A score from 27.5±7.1 (baseline) to 19.9±5.3 at 3 months (p<0.001). All HAM-A subscales exhibited uniform reductions (one-way ANOVA). HMSE scores were also improved from 89.3±18.6 (baseline) to 101.4±19.8 at 3 months (p=0.044). However, the mean HDI score, which was 34.9±28.5 at baseline and 26.8±25.9 at 3 months, appeared to be unaffected by paroxetine treatment (p=0.347).
The headache responder rate was 58.8% in this study (responders vs nonresponders = 14 vs 10) when all subjects were analyzed on an LOCF basis. The initial HAM-A scores did not differ significantly between the responder and nonresponder groups (responders 27.1, nonresponders 28.1; p=0.752). The final HAM-A scores at 3 months also did not differ between responders and nonresponders (responders 18.6±4.9, nonresponders 21.8±5.5; p=0.871).
Bivariate correlation analysis revealed no significant correlation between the reduction in headache frequency and the improvement in HAM-A score (Pearson's correlation coefficient -0.196, p=0.422; Fig. 2) Moreover, there was no significant correlation between headache frequency reduction and either HDI or HMSE
This open-label study investigated the preventive effect of paroxetine for migraine, and the correlation between its anxiolytic effect and this preventive effect. After the paroxetine treatment, there was a reduction in both the level of anxiety and the headache frequency. However, the reduction in anxiety level was not correlated with the reduction in the headache frequency. Accordingly, it is likely that at least two mechanisms contribute independently to the migraine-prophylactic and anxiolytic effects of paroxetine.
Anxiety is a well-known migraine-precipitating factor.6,16 Other psychiatric comorbidities, such as depression or panic disorder, have also been linked to migraine.17,18 Antidepressants such as TCAs, SSRIs, or SNRIs are effective in the prevention of migraine;10 however, the mechanisms underlying their actions have not been clearly defined. Given that antidepressants usually exert anxiolytic effects and that migraine attacks can be precipitated by anxiety,17,18 it can be readily hypothesized that the improvement in anxiety levels is related to the reduction in the frequency of migraine attacks.
The results of this study are not consistent with the above hypothesis. The observed reduction in HAM-A score induced by paroxetine demonstrates the efficacy of paroxetine in reducing the anxiety levels of migraineurs. However, a comparison of anxiety levels between paroxetine responders vs nonresponders showed that initial anxiety levels did not predict the outcome of headache responsiveness. In addition, the degree of improvement in anxiety scores was not correlated with the reduction in headache frequency. Accordingly, migraine prophylaxis by paroxetine appears to be independent of its anxiolytic effect, and is likely to be mediated through a direct modulation of the serotonergic system.10
Our previous study with buspirone, a serotonin (5-hydroxytryptamine, 5-HT) 1A agonist, also reported that the prophylactic effect on migraine is not related to the reduction in anxiety levels.19 Our two studies also suggest that the effected reduction in anxiety is not sufficient to prevent migraine.
The serotonergic system has been implicated in the pathogenesis of migraine disorder. The key evidence implicating 5-HT in migraine includes increased urinary excretion of 5-hydroxyindoleacetic acid, which is the main metabolite of serotonin, and a rapid fall in platelet 5-HT levels during a migraine attack.20,21 Moreover, intravenously injected 5-HT alleviates reserpine-induced headache.22 The main serotonergic systems in migraine pathophysiology are 5-HT1 and 5-HT2.24 There are three subtypes of 5-HT2 receptors: A, B, and C. Some migraine-prevention drugs, such as methysergide, cyproheptadine, and pizotifen, are potent 5-HT2B and 5-HT2C receptor antagonists, whereas metachlorophenylpiperazine, a 5-HT2B and 5-HT2C receptor agonist, can trigger migraine in susceptible individuals.24,25 The effect of paroxetine in migraine is likely to be attributable to the potentiation of these various serotonergic systems via the inhibition of selective 5-HT reuptake. However, there are controversial conclusions from past trials of SSRIs in migraines that SSRIs are no more efficacious than placebo in patients with migraine.10 Therefore, further studies are warranted.
One limitation of this study is its open-label design and the inclusion of only a small number of subjects. Given that a small sample size can cause false-negative results, the correlation between the headache responsiveness and the anxiolysis should be verified in a larger population. Furthermore, the efficacy of paroxetine in migraine should be investigated in placebo-controlled trials.
In summary, our results suggest that paroxetine is effective for both migraine prevention and anxiolysis in migraineurs with anxiety, and that its effect on migraine prevention is not secondary to its anxiolytic effect. Given that this study is based on small populations and is an open-label design, further studies are warranted to elucidate the discrete mechanisms involved in its actions, as well as to establish the controversial effects of SSRI in migraine.
Figures and Tables
Figure 1
Monthly headache frequency after paroxetine administration. Headache frequency decreased to 49.1% of the baseline level at 3 following treatment with paroxetine (*p<0.01 vs baseline, as assessed by paired t-test). mo = months.
References
1. Breslau N, Rasmussen BK. The impact of migraine: Epidemiology, risk factors, and co-morbidities. Neurology. 2001. 56:S4–S12.

2. Wang SJ, Fuh JL, Young YH, Lu SR, Shia BC. Prevalence of migraine in Taipei, Taiwan: a population-based survey. Cephalalgia. 2000. 20:566–572.

3. Sakai F, Igarashi H. Prevalence of migraine in Japan: a nationwide survey. Cephalalgia. 1997. 17:15–22.
4. Roh JK, Kim JS, Ahn YO. Epidemiologic and clinical characteristics of migraine and tension-type headache in Korea. Headache. 1998. 38:356–365.

5. Blau JN. Anxiety and depression in migraine. J R Soc Med. 1995. 88:59.
6. Wacogne C, Lacoste JP, Guillibert E, Hugues FC, Le Jeunne C. Stress, anxiety, depression and migraine. Cephalalgia. 2003. 23:451–455.

7. Saper JR. Saper JR, editor. Migraine: precipitating factors. Headache Disorders. 1983. Boston: John Wright;33–48.
8. Landy S, McGinnis J, Curlin D, Laizure SC. Selective serotonin reuptake inhibitors for migraine prophylaxis. Headache. 1999. 39:28–32.

9. d'Amato CC, Pizza V, Marmolo T, Giordano E, Alfano V, Nasta A. Fluoxetine for migraine prophylaxis: a double-blind trial. Headache. 1999. 39:716–719.
10. Moja PL, Cusi C, Sterzi RR, Canepari C. Selective serotonin re-uptake inhibitors (SSRIs) for preventing migraine and tension-type headaches. Cochrane Database Syst Rev. 2005. CD002919.

11. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004. 24:9–160.
13. Jacobson GP, Ramadan NM, Aggarwal SK, Newman CW. The Henry Ford Hospital Headache Disability Inventory (HDI). Neurology. 1994. 44:837–842.

14. Martin NJ, Holroyd KA, Rokicki LA. The Headache Self-Efficacy scale: adaptation to recurrent headaches. Headache. 1993. 33:244–248.

15. French DJ, Holroyd KA, Pinell C, Malinoski PT, O'Donnell F, Hill KR. Perceived self-efficacy and headache-related disability. Headache. 2000. 40:647–656.

16. Amery WK, Vandenbergh V. What can precipitating factors teach us about the pathogenesis of migraine? Headache. 1987. 27:146–150.

17. Breslau N, Davis CG, Andreski P. Migraine, psychiatric disorders, and suicide attempts: an epidemiologic study of young adults. Psychiatry Res. 1991. 37:11–23.

18. Merikangas KR, Angst J, Isler H. Migraine and psychopathology. Results of the Zurich cohort study of young adults. Arch Gen Psychiatry. 1990. 47:849–853.
19. Lee ST, Park JH, Kim M. Efficacy of the 5-HT1A agonist, buspirone hydrochloride, in migraineurs with anxiety: a randomized, prospective, parallel group, double-blind, placebo-controlled study. Headache. 2005. 45:1004–1011.

20. Curran DA, Hinterberger H, Lance JW. Total plasma serotonin, 5-hydroxyindoleacetic acid and p-hydroxy-m-methoxymandelic acid excretion in normal and migrainous subjects. Brain. 1965. 88:997–1010.

21. Anthony M, Hinterberger H, Lance JW. Plasma serotonin in migraine and stress. Arch Neurol. 1967. 16:544–552.

22. Kimball RW, Friedman AP, Vallejo E. Effect of serotonin in migraine patients. Neurology. 1960. 10:107–111.

23. Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International Union of Pharma cology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev. 1994. 46:157–203.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download